The structure of protein sets the foundation for its interaction with other molecules in the body and, therefore, determines its function. This article will cover the structural principles of proteins and how these can have an effect on the function of the protein.
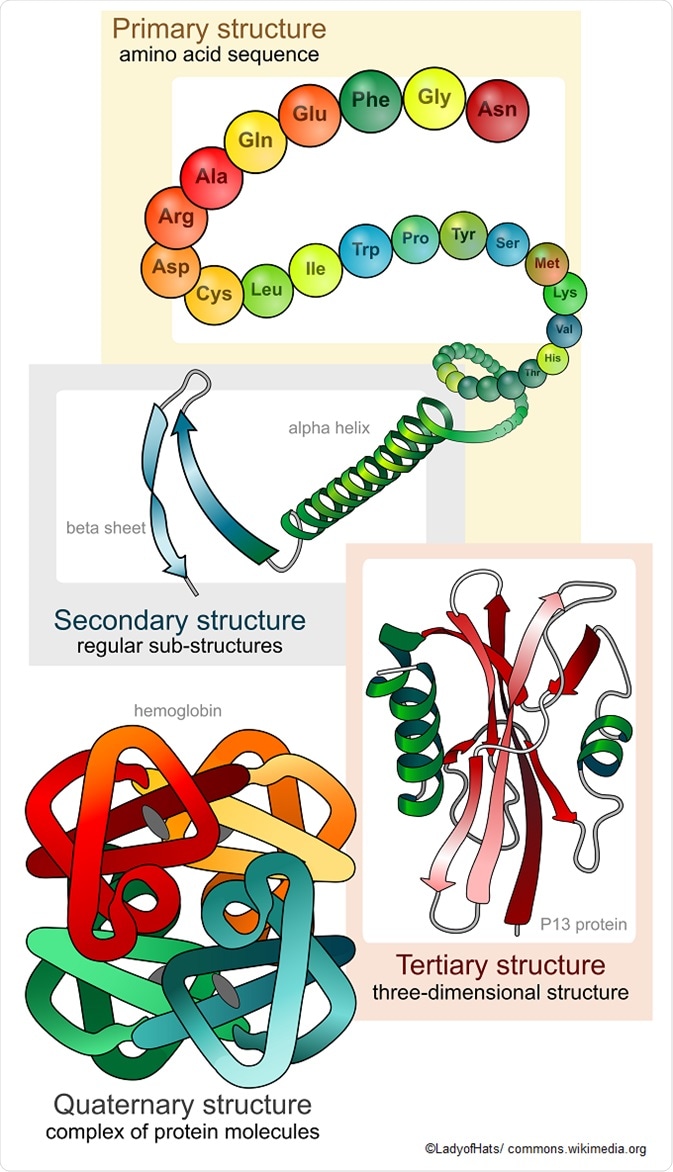
Primary protein structure
Proteins are made up of a long chain of amino acids. Even with a limited number of amino acid monomers – there are only 20 amino acids commonly seen in the human body – they can be arranged in a vast number of ways to alter the three-dimensional structure and function of the protein. The simple sequencing of the protein is known as its primary structure.
Secondary protein structure
The secondary protein structure depends on the local interactions between parts of a protein chain, which can affect the folding and three-dimensional shape of the protein. There are two main things that can alter the secondary structure:
- α-helix: N-H groups in the backbone form a hydrogen bond with the C=O group of the amino acid 4 residues earlier in the helix.
- β-pleated sheet: N-H groups in the backbone of one strand form hydrogen bonds with C=O groups in the backbone of a fully extended strand next to it.
There can also be a several functional groups such as alcohols, carboxamines, carboxylic acids, thioesters, thiols, and other basic groups linked to each protein. These functional groups also affect the folding of the proteins and, hence, its function in the body.
Tertiary structure
The tertiary structure of proteins refers to the overall three-dimensional shape, after the secondary interactions. These include the influence of polar, nonpolar, acidic, and basic R groups that exist on the protein.
Quaternary protein
The quaternary protein structure refers to the orientation and arrangement of subunits in proteins with multi-subunits. This is only relevant for proteins with multiple polypeptide chains.
Proteins fold up into specific shapes according to the sequence of amino acids in the polymer, and the protein function is directly related to the resulting 3D structure.
Proteins may also interact with each other or other macromolecules in the body to create complex assemblies. In these assemblies, proteins can develop functions that were not possible in the standalone protein, such as carrying out DNA replication and the transmission of cell signals.
The nature of proteins is also highly variable. For example, some are quite rigid, whereas others are somewhat flexible. These characteristics also fit the function of the protein. For example, more rigid proteins may play a role in the structure of the cytoskeleton or connective tissues. On the other hand, those with some flexibility may act as hinges, springs, or levers to assist in the function of other proteins.
Protein functions
Proteins play an important role in many crucial biological processes and functions. They are very versatile and have many different functions in the body, as listed below:
- Act as catalysts
- Transport other molecules
- Store other molecules
- Provide mechanical support
- Provide immune protection
- Generate movement
- Transmit nerve impulses
- Control cell growth and differentiation
The extent to which the structure of proteins has an impact on their function is shown by the effect of changes in the structure of a protein. Any change to a protein at any structural level, including slight changes in the folding and shape of the protein, may render it non-functional.
References
- https://www.ncbi.nlm.nih.gov/
- http://genome.tugraz.at/MolecularBiology/WS11_Chapter03.pdf
- https://www.boundless.com/
Last Updated: Jul 19, 2023