Supermolecules are formed from spontaneous assembly of linked molecular clusters of a unique composition, in a noncovalent manner, to form a larger final biological or chemical structure. The process of making a supermolecule is therefore thermodynamically favoured and involves a pre-designed assembly structure.
This is called supramolecular assembly. For example, apoferritin – a naturally forming protein that stores iron, is noncovalently composed of 24 linked protein subunits to form an almost spherical, octahedral and symmetrical shape.
![Host-guest complex within another host. This is a picture generated from crystal structure data reported by Anthony I. Day, Rodney J. Blanch, Alan P. Arnold, Susan Lorenzo, Gareth R. Lewis, and Ian Dance in Angewandte Chemie International Edition, Year 2002, Volume 41, Pages 275-277 doi: 10.1002/1521-3773(20020118)41:2<275::AID-ANIE275>3.0.CO;2-M. It shows a chlorine anion bound within a cucurbit[5]uril that both are bound within cucurbit[10]uril. The complex was described as a molecular gyroscope. It was made by myself and is free to be use by all. Image Credit: M stone Host-guest complex within another host. This is a picture generated from crystal structure data reported by Anthony I. Day, Rodney J. Blanch, Alan P. Arnold, Susan Lorenzo, Gareth R. Lewis, and Ian Dance in Angewandte Chemie International Edition, Year 2002, Volume 41, Pages 275-277 doi: 10.1002/1521-3773(20020118)41:2<275::AID-ANIE275>3.0.CO;2-M. It shows a chlorine anion bound within a cucurbit[5]uril that both are bound within cucurbit[10]uril. The complex was described as a molecular gyroscope. It was made by myself and is free to be use by all. Image Credit: M stone](https://www.news-medical.net/image-handler/picture/2018/10/Cucurbituril_gyroscope_AngewChemIntEd_2002_v41_p275_hires.jpg)
Host-guest complex within another host. This is a picture generated from crystal structure data reported by Anthony I. Day, Rodney J. Blanch, Alan P. Arnold, Susan Lorenzo, Gareth R. Lewis, and Ian Dance in Angewandte Chemie International Edition, Year 2002, Volume 41, Pages 275-277 doi: 10.1002/1521-3773(20020118)41:2<275::AID-ANIE275>3.0.CO;2-M. It shows a chlorine anion bound within a cucurbit[5]uril that both are bound within cucurbit[10]uril. The complex was described as a molecular gyroscope. It was made by myself and is free to be use by all. Image Credit: M stone
Supramolecular Assembly Within Biological Systems
DNA
A commonly occurring case of supramolecular assembly is a DNA structure involving a multi-layer of DNA, sandwiched between bilayer cell membranes in the presence of cationic liposomes (CLs). This is called: The lamellar 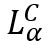 phase of CL-DNA complexes. The recent scantron X-ray diffraction studies show that the physical structure of this supramolecular assembly is different from the initially hypothesised “bead-on-a-string” structure proposed by Felgner et al in 1987 – which originally pictured a strand of DNA with CL’s attached to it. This new study shows a more layered approach to the supramolecular assembly, rather than a single, decorated strand.
phase of CL-DNA complexes. The recent scantron X-ray diffraction studies show that the physical structure of this supramolecular assembly is different from the initially hypothesised “bead-on-a-string” structure proposed by Felgner et al in 1987 – which originally pictured a strand of DNA with CL’s attached to it. This new study shows a more layered approach to the supramolecular assembly, rather than a single, decorated strand.
Naturally forming biomolecules
Certain proteins, oligonucleotides (a component of DNA), lipids, amongst other multimolecular complexes can all be found within biological systems. All of these are commonly used as inspiration for supramolecular chemists.
Chemical Uses of Supermolecules
Synthesis of polymers
The synthesis of polymers with specific characteristics has been an industrial science for many years. However, the concept of supramolecular polymers has only come about recently. Small molecular systems exhibit rubber-like, elastic behaviour which is an attractive concept for polymer chemists. For example, a mixture of ditopic and multitopic molecules (these are chemical structures which can interact with two or more molecules), forms a chemical network. This supramolecular assembly behave in a manner similar to resins or fibres – however, rubber-like behaviour has not yet been reported from this process.
Cross-links formation
Cross-links via hydrogen bonds provide the desired elastic effect. This system showed recoverable extensibility of up to 700%. However, unlike regular macromolecular rubbers, these supramolecular assemblies can self-heal by placing the two broken surfaces together at room temperature and waiting for the self-healing to take place.
Nanometer-scale structures
The supramolecular assembly of nanometer-scale structures is of great interest to nanoscientists – with liquid-to-liquid interfaces providing an alternative to regular formation of nanoparticles, nanocrystals, and viruses. This process can be applied to most surface types, including regular polymers.
Biomimetics
Supermolecules and supramolecular assembly is a perfect example of biomimetics – also known as the creation of a product, that is inspired by naturally occurring processes/structures. For example, most modern plane wings are based on similar structures seen in the wings of birds that are commonly seen gliding long distances (e.g. the albatross).
Many biological systems can be observed to use supramolecular assembly, in ways that assist biochemical functions. Chemists and biologists should work together to hypothesise and create new products and services – such as self-healing plastics.
Macroscopic self-assembly supramolecules
Last Updated: Dec 1, 2022