There are 20 canonical amino acids that are encoded by the genetic code of nearly all known organisms - there are only very few exceptions. In order to add novel building blocks to this existing repertoire, unique aminoacyl-tRNA synthetase and tRNA pairs are required, in addition to the specific amino acid codon and a source of the amino acid.
In terms of biological evolution, it is known that our ensemble of 20 amino acids has remained unchanged over a period of 3 billion years. This is demonstrated by the fact that all living organisms have adopted it.
While the possibility of amino acid to analog replacement could theoretically occur owing to the existence of analogs in nature, there is no evidence to date of such a phenomenon. There are no indications that any organism’s genetic code has ever had any of its canonical amino acids completely replaced by an analog.
However, it has been done experimentally with the help of bacteria and yeast. The first of such experiments were done using stable mutants of Bacillus subtilis that were selected to grow on 4-flurotryptophan (4FTrp). 4FTrp is an analog of tryptophan that contains indole.
Changes to the genetic code
The genetic code consists of 64 triplets of nucleotides called codons, with each codon, except 3, encoding one of the 20 amino acids. There is some redundancy in the code as most of the amino acids may be encoded by more than one codon.
Moreover, the code can be expressed as RNA or DNA codons with the former being used during translation (i.e. biosynthesis of proteins) after acquiring its sequence of nucleotides from the latter during transcription (i.e. copying of DNA into mRNA).
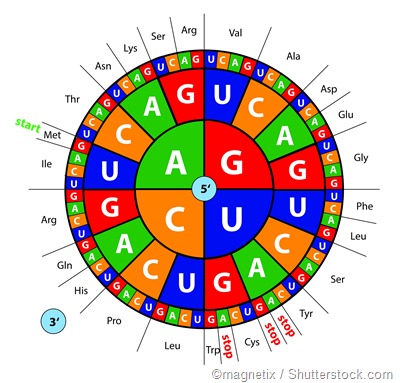
While it may be regarded as a common language shared between nearly all organisms, the genetic code is imperative for the interpretation of genes and the production of proteins. In addition to this, it precisely guides how the genome is put together via a series of fundamental and biochemical constraints.
This has allowed the code to be fairly refractory to change and enables it to shape how mutations that arise affect the evolution of the genome.
Changes anywhere within codon function must be tolerated on a genome-wide scale. This means that compensatory changes throughout the rest of the genome must occur if there are small changes to the genetic code.
Random mutagenesis is unlikely to cause such stable changes. However, contemporary feats in engineering are enabling the possibility of making rational changes to the genetic code.
Expanding the genetic code
To date, with the development of new orthogonal aminoacyl-tRNA synthetase and tRNA pairs, over 70 c (UAAs) have been added into E. coli, yeast and mammalian cells.
The orthogonal aminoacyl-tRNA synthetase and tRNA pairs are often engineered within other eukaryotic or bacteria cells and do not cross-talk with other endogenous pairs. However, they are functionally compatible with all components of the cellular machinery required to make proteins.
UAAs have been cotranslationally incorporated into proteins that have high efficiency and fidelity. The resulting incorporated amino acids tend to have features such as unique functional groups, special labels and properties and post-translational modifications.
The possibilities with engineering additional amino acids and adding to our existing canonical 20 amino acids are very promising. They will provide for powerful approaches in terms of probing protein function and structure both in vitro and in vivo. Likewise, they will allow for the generation of proteins with novel and/or enhanced properties.
NSF Grant Supports Chemist's Work to Expand Genetic Code
Further Reading
Last Updated: Feb 26, 2019