When the body becomes injured or unwell, there are many biochemical responses which are activated, including immune response, blood-clotting response, and inflammatory response. A multiprotein oligomer (a molecule consisting of many protein units), called the inflammasome, mediates this inflammatory response to injury/illness.
The inflammasome unit and its actions inside the cell. Image Credit: Ellepigrafica / Shutterstock
Inflammasome Structure
Scaffolding
The inflammasome consists of caspsase-1 enzyme, caspase-5 enzyme, Pycard/Asc, and NAPL1, a Pyrin domain-carrying protein which shares a structural homology with NODs (nucleotide-binding oligomerization domain-like receptors). The main scaffolding of inflammasome assembly is composed of filaments of Pyrin domains (the means of proteins chemically interacting with each other) and caspase recruitment domains.
Caspases
Caspases are enzymes that are part of and mediated by the inflammasome, are a family of endoproteases that are strongly linked with cell death and inflammatory response. The activation of these specific enzymes is strictly controlled by a few biochemical mediators, including the inflammasome. Once these enzymes are released, they gain catalytic energy through signalling events, including biochemical cascades. This specificity ensures the controlled destruction of cellular components that are no longer needed, and can then be used in other parts of the cell.
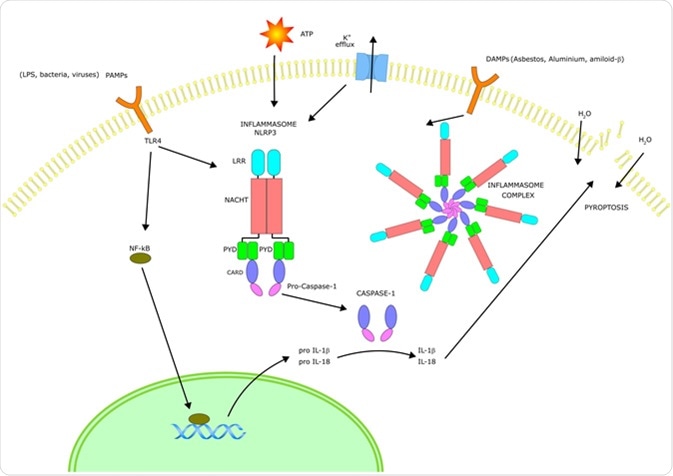
The Inflammasome signalling pathway
Mechanisms of the Inflammasome
A selection of germline-encoded pattern-recognition receptors are a major component of the inflammasome activation complex along with other receptors. Once in the presence of a certain stimuli (e.g. a specific pathogen cell-surface protein), the relevant receptors oligomerize to become a caspase-1-activation scaffold, which eventually induces the inflammatory response.
Inflammasome activation cascade
The inflammasome activation cascade depends on the type of inflammasome being exposed to the stimuli. Prominent inflammasomes include ASC, AIM2, and NAIP/NLRC4. AIM2, for example, directly binds to double-stranded DNA, its specific stimuli. The NLRP3 inflammasome can be activated with the widest range of stimuli compared to all other known inflammasomes which leads to a common theory that dissimilar agonists can induce further downstream reactions, which are sensed by the NLRP3 inflammasome.
Problems caused by the Inflammasome
Errors within inflammasome
If there is an error within certain inflammasome/related inflammatory receptors, it can cause diseases. For example, functional mutations within the NACHT domain of the NLRP3 inflammasome causes a selection of chronic, aseptic inflammatory diseases: Muckle-wells syndrome, familial cold autoinflammatory disorder (FCAS), and neonatal onset multi-systemic inflammatory disease/chronic infantile neurological cutaneous articular syndrome (NOMID/CINCA). These conditions are all related to the family of cryopyrin-associated periodic syndromes.
Gout
Gout is an inflammatory disease caused by monosodium urate crystal deposition in various tissues around the body. It causes clinical symptoms, such as acute monoarthritis. It leads to deposition of monosodium urate crystals inside joints, which triggers an acute inflammatory response by the NLRP3 inflammasome. Monosodium urate crystals can activate the NLRP3 inflammasome, both in vitro (in a lab), and in vivo (inside a patient’s body).
Treatment Strategies
A common method of treatment is use of therapies with Interleukin-1. This family of ligands and receptors are associated with both acute and chronic inflammatory disorders, with interleukin (IL) 1β being observed as a therapeutic target for local autoinflammatory diseases. The de-activation of IL-1β can result in a quick and sustained reduction of the severity of the disease and its symptoms.
Further Reading
Last Updated: Aug 11, 2022