Proteins play a plethora of roles within biological organisms critical to their optimal function and health. There are many different classes of protein that have been identified by scientists since their discovery and protein kinases are amongst them. This article will discuss the JNK protein, part of this class of protein.
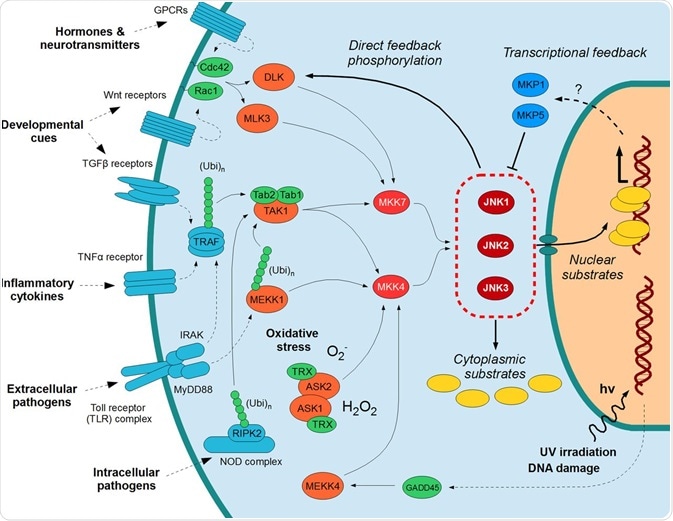
Image Credit: https://mmbr.asm.org/content/80/3/793
Protein Kinases
Protein kinases are biologically important enzymes involved in intracellular signaling functions. The largest family of enzymes encoded for, they catalyze the phosphorylation of target substrate proteins. Whilst much research has been performed concerning this class of enzyme over the past 20 years, many questions remain about them.
Scientists have a basic appreciation of the regulatory role that protein phosphorylation plays in intracellular signaling but there is much more research needed to completely understand this process. How it directly modifies protein function, affects signaling intermediaries, and how cellular behavior is thus modified largely remains a mystery. Knowledge of the process and how protein kinases, including the JNK protein, will help inform our understanding of health and disease at a cellular level.
The JNK Protein
The c-Jun N-terminal kinase (JNK) protein family of the Ser/Thr protein kinases has been the focus of intense study for the past two decades. Several studies have been performed covering general and specific aspects of these protein kinases, including their involvement in signaling in the brain and for cancer treatment strategies that inhibit JNK signaling. There are several attractive pharmaceutical applications for them due to their roles in disease development.
The JNK proteins belong to a class of proteins known as the MAPK (mitogen-activated protein kinase) family. They relay, amplify, and integrate signals from a diverse range of stimuli, both intra- and extracellular. Stress stimuli that are involved in activation of the JNK pathway include ultraviolet irradiation, inflammation, heat or osmotic shock, and damage to DNA.
JNK proteins consist of 10 isoforms which are derived from 3 genes. JNK1 has four, JNK2 has two, and likewise, there are two JNK3 isoforms. Depending on how the 3’ coding region of the corresponding mRNA is processed, the genes are expressed as either 46kDa or 55 KDa protein kinases. There appears to be no functional difference between the isoforms.
Distribution of the different JNK isoforms is as follows:
- JNK1/JNK2 – All tissues and cells
- JNK3 – Primarily the brain, but also in the heart and testes.
The function of JNK Proteins
As has been mentioned, various stress stimuli work to activate the JNK pathway. One way this occurs is via disruption of protein phosphatase enzymes; these normally inhibit JNK activity and proteins linked to its activation.
JNK proteins can associate with scaffold proteins, JIP (JNK interacting proteins) as well as upstream kinases following activation. They modify the activity of proteins at the mitochondria or within the nucleus. JNK activates several downstream molecules, including ATF2, SMAD4, HSF1, and c-Jun. It inhibits downstream molecules including NFATC1 and STAT4. It is through the activation and inhibition of downstream molecules such as these that JNK proteins regulate cellular activity.
Recent research has shown that, through phosphorylation and activation of the ubiquitin ligase Itch, JNK1 regulates Jun protein turnover.
JNK Proteins and Cancer
Since the early 1990s, there has been a series of studies that have found that JNKs can transform several oncogenes such as Ras, Met, and c-fos. Indeed, JNK signaling seems to contribute to cellular transformations that lead to the development of cancer.
For example, the downstream product c-Jun is involved in Ras-induced transformation and is involved in liver cancer formation in mice models. In studies on intestinal cancer in mouse models, mice that express a non-phosphorylatable mutant form of c-Jun showed a marked reduction in polyp development. This suggests that c-Jun’s oncogenic function is dependent upon JNK phosphorylation.
Several studies have reported that hyperactivated JNK proteins are present in multiple tissue samples and cancer cell lines and depleted levels of individual JNKs can suppress the genesis of tumors, dependent upon tissue specificity. One study found that activation of JNK1 (but not JNK2) was involved in the proliferation of tumor cells in primary hepatocellular carcinomas. Overactivation has also been linked to tumor-promoting activity in lung cancer due to smoking.
However, there is also evidence implicating JNK proteins in tumor suppression. Studies in mice models have suggested that this is due to JNK proteins’ apoptotic functions which are connected with the mitochondrial pathway. JNK2 has also been shown to phosphorylate p53, a tumor suppressor on Ser6. JNK1 and JNK2 act as both tumor promoters or suppressors in different cancers.
Other Roles
JNK proteins play several other important roles in the body. These include DNA repair (JNK proteins are involved in one of the early steps of double-strand break repair) and diabetes. Although still poorly understood, the importance of these proteins cannot be understated when it comes to the healthy functioning of organisms.
Drosophila flies that express mutations that regulate and augment JNK proteins have been shown to live longer than wild flies. This is due to reduced oxidative damage accumulation over their lifespans. Caenorhabditis elegans, a roundworm, can live up to 40% longer if wild-type JNK1 is amplified.
In Conclusion
JNK proteins have been shown to play several vital roles in organisms, involved in the regulation of cancer, inflammatory response, DNA repair, and even aging. More research is needed to elucidate the specific mechanisms of this important regulatory and signaling pathway and will open up the possibility of several new classes of novel therapeutics for a variety of debilitating conditions.
Sources
- Bubici, C & Papa, S (2014) JNK signaling in cancer: in need of new, smarter therapeutic targets Br J Pharmacol. 171 (1) pp. 24-37 (Accessed 30th July 2020) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3874694/
- Sueng Wook Oh et al. (2005) JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16 PNAS 102 (12) pp. 4494-4499 (Accessed 30th July 2020) https://www.pnas.org/content/102/12/4494
- Wang, M.C. Bohmann, D & Jasper. H. (2005) JNK Extends Life Span and Limits Growth by Antagonizing Cellular and Organism-Wide Responses to Insulin Signaling Cell 121 (1) pp. 115-125 (Accessed 30th July 2020) https://doi.org/10.1016/j.cell.2005.02.030
Last Updated: Sep 30, 2020