May 18 2009
Ellman International, Inc. announced today that the Food and Drug Administration (FDA) has granted clearance to Pelleve, a skin tightening system for the non-ablative treatment of mild to moderate facial wrinkles and rhytids for skin phototypes I-IV.
The clearance was granted based on clinical data demonstrating that a single treatment with the high frequency radiowave device can safely and effectively tighten and improve the appearance of skin on the face through six months.
"Today's FDA clearance of the Pelleve system represents a very exciting step in the introduction of next-generation high frequency radiowave technology to physicians and patients in the United States," said Rick Epstein, CEO of Ellman International, Inc. "Pelleve offers safe, non-invasive facial rejuvenation with no need for a local anesthetic and minimal discomfort -- a significant evolution from previous-generation technologies in this class."
Pelleve uses the advanced radiowave technology of the Ellman International Surgitron(R) Dual RF S5 and a proprietary Pelleve handpiece to precisely deliver energy through the skin to the dermal tissue beneath without damaging the epidermis. This gentle heating of the deeper dermal tissue induces collagen denaturization and contraction. As the dermal tissue recovers, new collagen synthesis occurs, which creates a tightening effect. The result is a noticeable improvement in skin quality and appearance with minimal side effects and healing time for patients.
In a clinical trial conducted to determine the effectiveness of the Pelleve system, 83 women and 10 men were given a single treatment with the Pelleve handpiece. The procedure was performed in an ambulatory (outpatient) setting with no need for skin cooling products or anesthesia and took an average of 15 to 20 minutes, depending on size of the area. More than 87% of patients showed measurable and immediate positive results in a blinded assessment of skin laxity and wrinkle improvement with continued response at six months after treatment. Patients were typically able to return to work and social activities immediately after treatment. Of the study population, two patients experienced small abrasions that healed within three days.
"Our research clearly demonstrates the power of the Pelleve Skin Tightening System. With a single Pelleve treatment, over 87% of our patients experienced an improvement in skin laxity and fewer overall facial wrinkles at six months. The Pelleve Skin Tightening System is an effective, non-invasive, economical and safe tool," said Dr. Antonio Rusciani, Division of Dermatology, Plastic and Reconstructive Surgery at the University of Rome in Rome, Italy.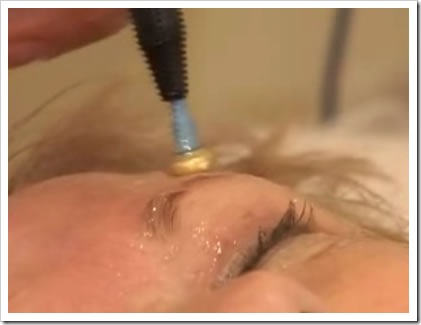
Ellman's skin tightening technology has been marketed with a CE Mark in many countries since 2005 under the name Radiage. "We have seen positive results in many patients outside of the United States. Because this unique technology does not require anesthesia or cooling of the skin during the procedure, the physician can safely achieve the look of lifted, tighter skin without surgery, resulting in a more youthful appearance for the patient," said Dr. Kai Rezai, a noted dermatologist and Radiage user from Munster, Germany.
Designed to work in conjunction with the Surgitron Dual RF S5 platform, Ellman's patented 4.0 MHz radiowave unit, Pelleve handpieces represent just one of hundreds of accessories available for office and clinic environments. The Surgitron Dual RF S5 provides maximum control in precision cutting and energy delivery, with more versatility than other energy-based technologies. "Ellman's proven product platform enables medical professionals to perform surgical procedures that produce less tissue damage and pain than the competition. Adding Pelleve offers tremendous value to physicians, as customers ask for the latest in cosmetic correction from their dermatologists, plastic surgeons and other healthcare professionals," said Epstein. Pelleve will be introduced to the U.S. market this month through dermatologists, plastic surgeons, ENTs and other cosmetic surgery clinics.