Jun 9 2009
Impliant, Inc., a developer of novel spine arthroplasty alternatives to fusion surgery, today announced that the European Patent Office has confirmed the validity of one of its key patents following an Opposition by Archus Orthopedics, Inc. (Redmond, WA) that concluded with oral proceedings on May 12, 2009 in Munich, Germany.
This patent (EP1578314 B1), originally issued to Impliant on May 30, 2007, involves among others the company's flagship product, the TOPS System, a Total Posterior Arthroplasty device designed to treat lumbar spinal stenosis with or without facet arthrosis and spondylolisthesis. Archus challenged the patent on the grounds of being too broad, but the European Patent Office rejected the opposition and upheld every claim of the patent.
Impliant also announced that it has been granted two additional patents in the United States. The first (US 7537613), granted on May 26, 2009, is another patent that directs to the company's TOPS System. The second (US 20050197705), already a patent in Europe (EP1646338), entails a unique translateral spinal disc replacement.
"We are very pleased by the outcome of these procedings," said Todd Potokar, President and CEO of Impliant. "The successful defense of one of our main patents in Europe coupled with the addition of two new patents in the U.S. further strengthens our robust IP portfolio."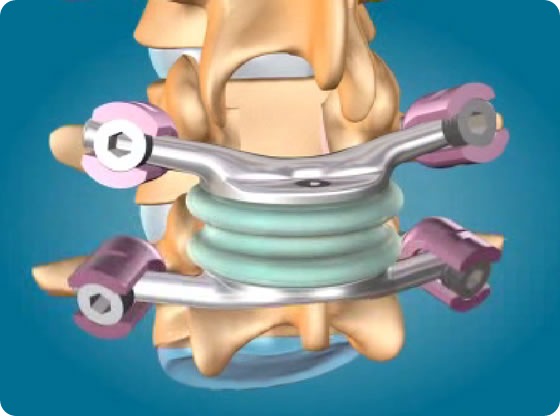
Impliant is applying cutting-edge materials and crossbar biomechanical techniques to develop a new class of spine arthroplasty devices that target over 40% of the patients worldwide who undergo fusion surgery and could benefit from a Total Posterior Arthroplasty solution.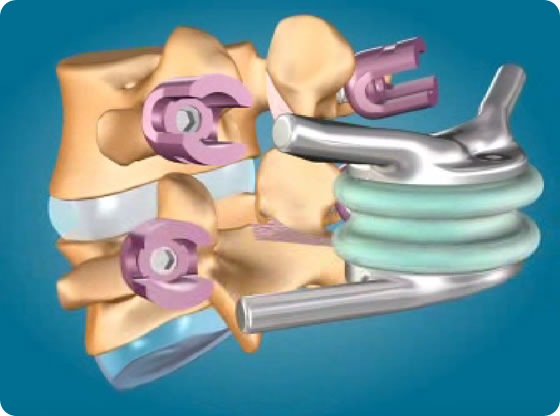
Impliant's TOPS System, a mobile posterior device, is designed to stabilize but not fuse the L3-4 or L4-5 vertebral level to alleviate pain stemming from spinal stenosis with or without degenerative facet arthrosis, and spondylolisthesis. Following a laminectomy and medial facetectomy, the device is affixed to the spine via four pedicle screws using a standard posterior surgical approach. Impliant believes that the TOPS System could benefit over 500,000 patients worldwide undergoing spinal fusion surgery each year. The TOPS System is not approved for sale in the United States.