A technique for producing natural killer T (NKT) cells, known for their role in suppressing tumor growth, has been successfully demonstrated for the first time using induced pluripotent stem (iPS) cells. Developed by researchers at RIKEN, Japan’s flagship research institution, the technique opens the door to effective new cell-targeted treatments for cancer.
Through their production of TH1 cytokines, NKT cells play an essential role in innate immune responses, protecting against tumors and virus-infected cells. Yet while clinical trials have shown that injection of the glycolipid α-GalCer can effectively activate an NKT cell antitumor response, most cancer patients have very few NKT cells, which has prevented the wider application of this therapy.
Induced pluripotent stem (iPS) cells, “all-purpose” cells capable of differentiating into any type of cell, present a potential solution to this shortage of NKT cells, but with a catch: in lymphocytes, rearrangement of genes during differentiation drastically diminishes the effectiveness of conventional iPS cell generation techniques, resulting in only a small portion of cells with the desired antitumor function.
To overcome this problem, the research team used mature NKT cells that had already undergone gene rearrangement to derive their iPS cells. As reported in the July issue of the Journal of Clinical Investigation, these iPS cells enabled them to generate large numbers of NKT cells that secrete abundant IFN-γ, a TH1 cytokine that activates antitumor functions. When tested using a mouse model, the cells successfully reproduced the effects of natural NKT cells and suppressed tumor growth, validating the approach and setting the stage for powerful clinical applications in cancer therapy.
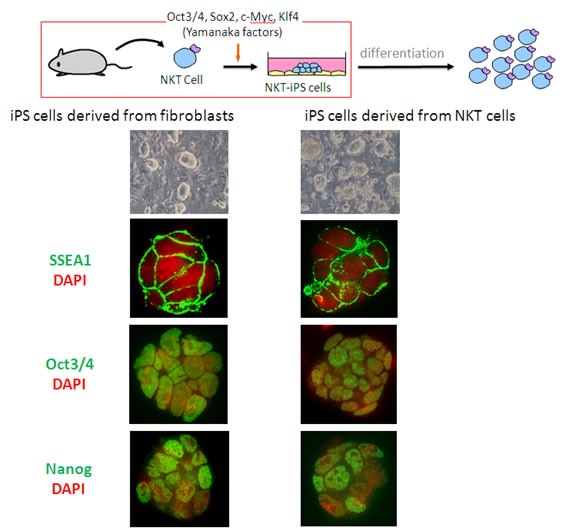 |
| Figure 1: Generation of iPS cells harboring NKT cell-specific rearranged T cell receptor loci. The stably established lines exhibited ES cell-like morphology and expressed the same proteins (SSEA, Oct 3/4 and Nanog) as iPS cells derived from fibroblasts. |
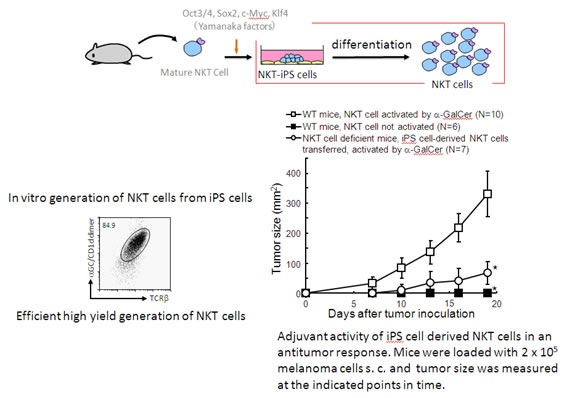 |
| Figure 2: In vitro generation of NKT cells with desired function from iPS cells. |
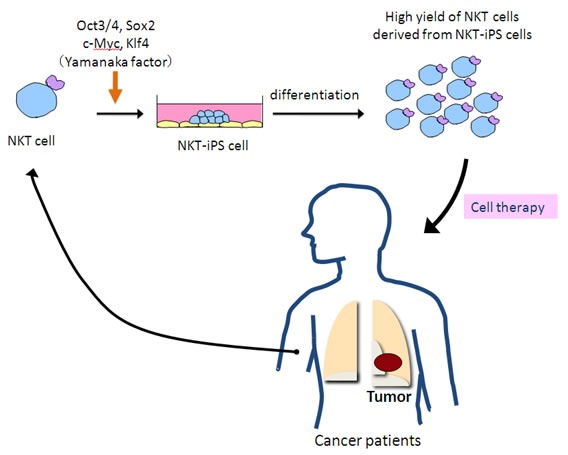 |
| Figure 3: Proposal: NKT Cell Adjuvant Immunotherapy. Our study not only provides definite proof that terminally differentiated NKT cells can be directly reprogrammed to pluripotency but also increases the availability of mature NKT cells with desired functions to establish optimal NKT cell therapy. |