Colorectal cancer (CRC) is the third most common cancer in men and women in the USA, with a new diagnosis made every 3.5 minutes and an associated death every 9 minutes.1
The incidence of CRC in the USA in 2002 was 169 000, and is projected to rise to about 196,000 by 2018.2 CRC is relatively rare in younger people (1:1406 under the age of 39 years), with the incidence rising sharply after the age of 40 years (1:123 aged 40–59 years), and becoming higher still after age 60 years (1:73 among those aged 60–69 years; 1:21 among those aged over 70 years).3
If diagnosed early, however, CRC is potentially curable, allowing some patients to make a full recovery.4 The 5-year mortality rate is approximately 40%, but survival improves substantially if the cancer is diagnosed before it has metastasized.1 Unfortunately, many patients often only become symptomatic once the disease is advanced and metastatic.5,6
Colorectal cancer metastases affect approximately 50% of patients with CRC.7 They may present either at the time of diagnosis (synchronous) or subsequently (metasynchronous) and surgery offers the only potential for cure in these patients.
Up to 90% of patients with metastases are initially non-resectable;7 however, advances in neoadjuvant therapies have expanded the potential for converting some patients to resectable status, thus increasing the proportion of patients able to achieve long-term survival.7
Targeted therapies for metastatic CRC
Epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein that, when stimulated, increases tumor cell proliferation, invasion, and migration, and the inhibition of apoptosis (programmed cell death). This protein has been detected in a wide variety of cancers and its overexpression may be a factor for poor prognosis.8,9 One reason why EGFR is such an attractive site for intervention is that it is present in 30–100% of solid tumors.10
To date, three targeted agents have been approved by the US Food and Drug Administration for the treatment of metastatic CRC (mCRC):
- Avastin® (bevacizumab; Genentech/Roche)
- Erbitux® (cetuximab; ImClone Systems Inc./Bristol-Myers Squibb/Merck Serono,
- Vectibix® (panitumumab; Amgen).
Avastin® targets the vascular endothelial growth factor receptor (EGFR) A ligand, whereas both Erbitux® and Vectibix® target EGFR by blocking signal pathways at the cell membrane.
Clinical efficacy of the EGFR antagonists
Erbitux® – a chimeric mouse-human monoclonal antibody (MAb) – was the first anti-EGFR MAb to be approved for clinical use in patients with mCRC. It is has been evaluated primarily in combination with chemotherapy,11,12 but also as monotherapy.11,13,14
“For drugs with a similar mechanism of action, first-to-market status has historically been the most important factor determining commercial success”
| Table 1: Regulatory history of Erbitux in CRC, 2004–08 |
| Year | Market | Approval/line extension |
| 2004 | US | Approved in combination with irinotecan to treat EGFR-expressing mCRC patients who are refractory to irinotecan-based chemotherapy and as a single agent in EGFR-expressing mCRC patients after failure of irinotecan-based regimens |
| 2005 | 5EU | Approved in combination with irinotecan for the treatment of patients with EGFR-expressing mCRC after failure of irinotecan-based therapy |
| 2007 | US | Label was extended to include overall survival data as a single agent in EGFR-expressing mCRC after failure of both
irinotecan- and oxaliplatin-based regimens |
| 2008 | 5EU | Label was broadened to include first-line use in patients with EGFR-expressing, KRAS wild-type disease |
| 2008 | Japan | Approved for the treatment of patients with EGFR-positive, curatively unresectable (inoperable), advanced or recurrent CRC. The approval allowed for the use of cetuximab with irinotecan in the second and further lines of mCRC |
| 2009 | US | Label amended to prevent the use of Erbitux® in patients whose tumors have KRAS mutations in codon 12 or 13 |
| 2010 | Japan | Approved as a first-line treatment, in combination with chemotherapy, for patients with EGFR-expressing, curatively unresectable (inoperable), advanced or recurrent mCRC carrying the KRAS wild-type gene |
| 5EU = five European markets (France, Germany, Italy, Spain, UK)
CRC = colorectal cancer; EGFR = epidermal growth factor receptor; mCRC = metastatic colorectal cancer |
| Source: Datamonitor |
Vectibix® – a fully human MAb – has shown efficacy as monotherapy in patients with mCRC resistant to chemotherapy.15 Ongoing combination chemotherapy trials in earlier lines of treatment have reported acceptable interim safety data.
| Table 2: Regulatory history of panitumumab in CRC, 2006–08 |
| Year | Market | Approval/line extension |
| 2006 | US | Approved for the third-line treatment of mCRC |
| 2007 | 5EU | Received conditional approval for use in metastatic patients whose tumors express EGFR and who do not have a genetic mutation in KRAS |
| 2008 | Japan | Takeda submitted an NDA for mCRC in Japan |
| 2009 | US | Label amended to prevent use of Vectibix in patients whose tumors have KRAS mutations in codon 12 or 13 |
| 2010 | Japan | Launch in Japan |
| 5EU = five European markets (France, Germany, Italy, Spain, UK)
CRC = colorectal cancer; EGFR = epidermal growth factor receptor; mCRC = metastatic colorectal cancer; NDA = New Drug Application |
| Source: Datamonitor |
Monotherapy with the EGFR antagonists
Both Erbitux® and Vectibix® have shown modest efficacy in two large randomized phase III trials in patients pretreated with a flouropyrimidine, oxaliplatin, and irinotecan.13,15 Response rates were 8% for Erbitux® and 10% for Vectibix® when compared with best supportive care (BSC).13,15 A significant improvement in progression-free survival (PFS) was seen with both Erbitux® (hazard ratio [HR]: 0.68, 95% confidence interval [CI]: 0.57 to 0.80, p <0.001) and Vectibix® (HR: 0.54, 95% CI: 0.44 to 0.66, p <0.0001).
In 2007, new data emerged demonstrating that Erbitux® monotherapy improved overall survival (OS) to 6.1 months versus 4.6 months in patients treated with BSC.13 This is still considered an important differentiator between the two EGFR MAbs, as Vectibix® was approved by only demonstrating a limited improvement in PFS versus BSC.
There are still currently no data demonstrating that Vectibix® offers any statistically significant improvements in survival. This lack of impact on OS relative to BSC suggests that Vectibix® is not as potent as Erbitux®, a perception that likely continues to be held among clinicians and one which has likely restricted the acceptance and subsequent uptake of Vectibix® compared to Erbitux® in clinical practice.
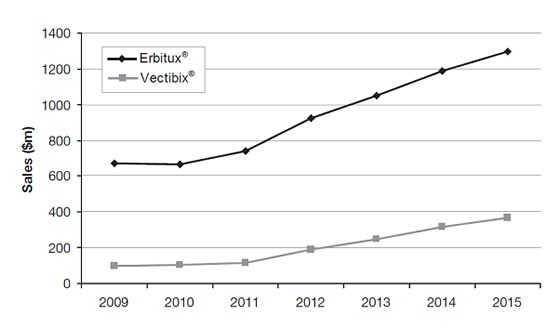
The clinical development timeline of both drugs, and the significant data advantage of Erbitux® over Vectibix®, can be graphically illustrated by their respective sales forecast over the next 5 years as shown in the Figure below.
Figure: US sales forecasts for Erbitux® and Vectibix® ($ m), 2009–15. Source: Datamonitor
Combination therapy: the EGFR antagonists with chemotherapy
Erbitux®
The EPIC trial12 showed that Erbitux® in combination with irinotecan was significantly better than irinotecan alone in terms of PFS (4 vs 2.6 months; HR: 0.692, 95% CI: 0.617 to 0.776, p ≤0.0001) and response rate (16.4% vs 4.2%, p <0.0001).
In 2007, data from the CRYSTAL study16 were presented at the American Society of Clinical Oncology (ASCO) meeting. These demonstrated an improvement in PFS in the first-line treatment of mCRC.
Erbitux® met all study endpoints by improving PFS from 8.0 to 8.9 months (p=0.036), and overall response from 38.7% to 46.9% (p=0.005), and reducing the risk of disease progression by 15%.
In an analysis of the CRYSTAL and OPUS studies, the addition of Erbitux® in the first-line setting to FOLFIRI (leucovorin, 5-flourouracil, irinotecan) and FOLFOX (leucovorin, 5-flourouracil, oxaliplatin) chemotherapy, respectively, was shown to significantly improve overall response, PFS and OS compared with chemotherapy alone.17
Furthermore, in the CRYSTAL study, patients with the wild-type KRAS gene showed a significant improvement in PFS with combination therapy compared with FOLFIRI alone. For all efficacy endpoints, including survival, KRAS status was confirmed as a predictor of treatment outcome in patients with mCRC receiving first-line Erbitux® and FOLFIRI. This landmark study was the first to establish the concept of personalized therapy as clinically meaningful in the treatment of mCRC.
In another study by Moosman et al,18 Erbitux® was shown to be well tolerated and efficacious in combination with both XELIRI and XELOX in patients with mCRC.
Vectibix®
The PRIME trial19 evaluated the efficacy and safety of Vectibix® in combination with FOLFOX4 versus FOLFOX4 alone as the first-line treatment of mCRC by tumor KRAS status. Combination therapy was shown to be significantly better than FOLFOX4 alone in terms of PFS (median: 9.6 vs 8.0 months; HR 0.80, 95% CI: 0.66 to 0.97, p=0.02), A non-significant increase in OS was also observed for combination therapy versus FOLFOX4 alone (median 23.9 vs 19.7 months, HR 0.83, 95% CI: 0.67 to 1.02, p=0.072).
In a study by Metges et al,20 Vectibix® was given to 32 patients who did not respond to Erbitux®, with a clinical benefit observed in 72.7% of patients (response rate: 54.5%), which strongly suggests that Vectibix® may be more effective in patients who have previously failed Erbitux® therapy.
The phenomenon of treatment resistance has been observed in many therapeutic approaches in cancer treatment and this data suggests that Vectibix® would be best reserved for patients who are resistant to Erbitux® treatment; i.e. in the third-line setting.
As Vectibix® is licensed as a monotherapy, this prevents physicians from using it in combination with chemotherapy, which is the preferred treatment option in the third-line setting.
“I do not see a lot of point in using Vectibix®…If you think at this stage that patients are still fit enough for some treatment, then they are usually fit for some chemotherapy with Erbitux®. Therefore, I have not used panitumumab monotherapy in any patients.”
US key opinion leader
| Table 3: Phase III study in combination with FOLFOX/FOLFIRI for first-line setting of mCRC (KRAS wild-type) |
| Study | Agent | Regimen | OS | PFS | RR | R0 |
| CRYSTAL16 | Erbitux® | FOLFIRI | YES | YES | YES | YES |
| OPUS21 | Erbitux® | FOLFOX | NO | YES | YES | YES |
| PRIME19 | Vectibix® | FOLFOX | NO | YES | NO | - |
| mCRC = metastatic colorectal cancer; OS = overall survival; PFS = progression-free survival; R0 = surgical resection; RR = response rate |
As can be seen from these summaries, the amount of Erbitux® data is far more extensive than that for Vectibix®, in terms of patient numbers, therapeutic experience, and clinical outcomes. And this is directly reflected in oncologists’ prescribing behavior, as explained later in this article.
“Erbitux® was earlier to market and there’re better studies. I have not seen any data that one is better than the other, so therefore I’m going to go by the volume of data for Erbitux®.”
US clinical oncologist
Safety issues with EGFR antibodies
Patients treated with Erbitux® or Vectibix® report similar adverse events with relative and equivalent frequency.13,15 Hypomagnesemia is relatively common with Erbitux® (48%) and Vectibix® (39%). Infusion reactions occur with both Erbitux® and Vectibix®.22,23
In a post hoc pooled analysis of the phase III CRYSTAL and phase II MABEL studies, infusion-related reactions were reduced to 1% when antihistamines and corticosteroids were used prophylactically.24 In terms of severity, grade 3–4 skin reactions were more common with Vectibix® (16%) compared with Erbitux® (12%).
From a mechanism perspective, EGFR inhibition does inevitably trigger these skin reactions (usually plantar and planar sites); however, these are expected and accepted as ‘normal’ at their usual low grade by oncologists and patients.
Patients taking both agents also reported fatigue, nausea, and vomiting, with the incidence being slightly higher with Erbitux®.
It has been suggested that Vectibix®, a fully human MAb, may have one minor potential advantage over Erbitux®: the latter is a chimeric MAb and in theory has an increased potential to cause a severe allergic response during infusion.21-23 However, this has no substantive basis in the ‘real world’ clinical setting and thus is of negligible concern to oncologists. Regardless, the risk of any allergic reaction can be greatly minimized by the administration of acetaminophen and an antihistamine prior to infusion of Erbitux®.22,24 This simple preventative measure negates any perceived benefit provided by Vectibix®.22,25
“I don’t tend to use Vectibix® because there are no data for it in combination with chemotherapy [as there are for Erbitux®]. So, the only time I would use Vectibix® would be in patients who have a reaction to Erbitux®.”
US key opinion leader
Prescribing patterns in the USA
In the USA, Erbitux® is much more widely used than Vectibix®. According to a 2010 surveyconducted at ASCO Gastrointestinal Cancer Symposium in 108 respondents (102 oncologists, 6 oncology nurses), there were several clear differentiating factors that could be obtained from the responses as to the respective use of Erbitux® and Vectibix®.
Predominant reasons cited for using Erbitux® (vs Vectibix®) can be shown in the Figure below:
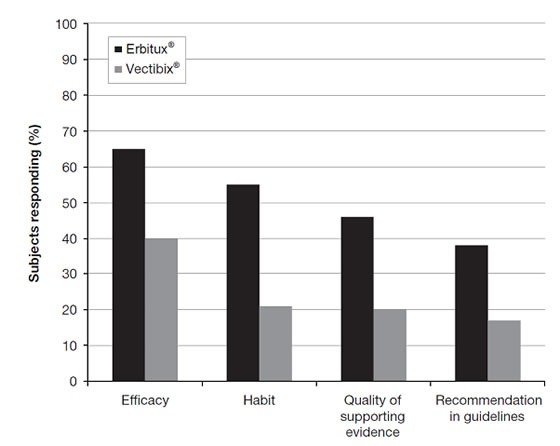
An overwhelming majority of respondents said that their first choice of EGFR therapy was Erbitux® (89% vs 10% with Vectibix®), with a substantial proportion citing their use of Erbitux® in second-line therapy (68.5% vs 7.4% with Vectibix®).
Avastin® remains the preferred first-line therapy of choice (82%), although unlike Vectibix®, Erbitux® is licensed as first-line therapy in Europe.
The combination of efficacy and habit are hugely important prescribing drivers in that they combine the two pillars that every clinician evaluates when considering treatment – clinical data and personal experience.
“The reason I tend to use Erbitux® is that I started with it, my nurses are comfortable with it…we know how to administer it, we know what to expect”
US clinical oncologist
This survey lends further weight to the feeling of trust and familiarity among US physicians with Erbitux® significantly restricting the uptake of Vectibix® in mCRC, which has become ‘niched’ as third-line monotherapy in patients unable to tolerate the irinotecan–Erbitux® combination.5,6
Role of KRAS mutations in improving response rates and clinical use
Retrospective analyses of numerous clinical trials have shown that Erbitux® and Vectibix® have significant efficacy in patients with CRC and wild-type KRAS mutations. These data provide a solid evidence base to support KRAS genotyping to identify suitable patients and predict response to EGFR treatment. This has the dual advantage of being beneficial to the patient in being administered a tailored treatment that will work, and to healthcare systems and resources, by providing a more cost-effective approach that could be achieved without the use of biomarkers to guide therapy.
In addition, the effect of mutations has been more extensively investigated with Erbitux® than Vectibix®. Whilst both agents have negligible clinical activity on tumors with KRAS mutations, Erbitux® does, however, have positive data with BRAF mutations, which is another positive differentiator from Vectibix® at this time.
Cost-effectiveness
By demonstrating the clinical value of Erbitux® to the UK’s cost-effectiveness watchdog, the National Institute for Health and Clinical Excellence (NICE), in 2009 Merck Serono successfully overturned an initial negative opinion for Erbitux® using data from retrospective KRAS analyses.
NICE’s U-turn highlights the future importance of the ability to select and treat patients who are most likely to respond to treatment with Erbitux®, particularly as other healthcare authorities in the six other major markets (USA, Japan, Germany, France, Italy, and Spain) are likely to follow NICE’s decision, citing the need for increased cost-effectiveness from these expensive treatment regimens.
In purely monetary terms, the results of a study presented at ASCO's 2009 Gastrointestinal Cancers Symposium suggested that the use of predictive KRAS testing to select patients for treatment with Erbitux® would theoretically save $740 million each year in the USA.25 Although this theoretical saving is artificially inflated (because the statistical parameters define the market penetration of Erbitux® as 100%), it nevertheless suggests that the cost of routinely using diagnostics is insignificant in comparison to the wasted cost of an otherwise blindly administered therapy that is unlikely to work in the majority of patients with mCRC.
Summary
As the first anti-EGFR MAb to be approved for use in patients with mCRC, the clinical experience and scientific data gathered for Erbitux® are greater than that for Vectibix®.
Current guidelines recommend the use of Erbitux® over Vectibix® in mCRC, primarily based on the extensive and much larger volume of robust positive data available for Erbitux®.
Prescribing patterns in the USA and Europe largely follow as a result of the implicit trust that such recommendations instill in oncologists. Further, the cost-effectiveness ‘hurdle’ has been jumped using KRAS mutational testing in addition to pricing strategy considerations, allowing as many potentially responsive mCRC patients to benefit from Erbitux® as possible. As for Vectibix®, although it no doubt has equivalent efficacy in certain clinical endpoints, it has currently been ‘niched’ into the third-line setting as it still lacks critical supporting data.
References
1 American Cancer Society. Colorectal Cancer Facts and Figures 2008-2010. Atlanta, GA: American Cancer Society, 2010.
2 Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74-108.
3 Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43-66.
4 Lloyd JM, McIver CM, Stephenson SA, Hewett PJ, Rieger N, Hardingham JE. Identification of early-stage colorectal cancer patients at risk of relapse post-resection by immunobead reverse transcription-PCR analysis of peritoneal lavage fluid for malignant cells. Clin Cancer Res 2006; 12: 417-23.
5 National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. NCCN Clinical Practice Guidelines in Oncology 2010; Version 1.2011.
6 National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. NCCN Clinical Practice Guidelines in Oncology 2010; Version 1.2011.
7 Adam R, Haller DG, Poston G, et al. Toward optimized front-line therapeutic strategies in patients with metastatic colorectal cancer--an expert review from the International Congress on Anti-Cancer Treatment (ICACT) 2009. Ann Oncol 2010; 21: 1579-84.
8 Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995; 19: 183-232.
9 Spano JP, Lagorce C, Atlan D, et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol 2005; 16: 102-8.
10 Ciardiello F, Tortora G. Anti-epidermal growth factor receptor drugs in cancer therapy. Expert Opin Investig Drugs 2002; 11: 755-68.
11 Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337-45.
12 Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 2311-9.
13 Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007; 357: 2040-8.
14 Saltz LB, Meropol NJ, Loehrer PJ, Sr., Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004; 22: 1201-8.
15 Van CE, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007; 25: 1658-64.
16 Van Cutsem E, Nowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer: the CRYSTAL trial. J Clin Oncol 2007; 25(suppl): 4000.
17 Bokemeyer C, Kohne C, Rougier P, Stroh C, Schlichting M, Van Cutsem E. Cetuximab with chemotherapy (CT) as first-line treatment for metastatic colorectal cancer (mCRC): analysis of the CRYSTAL and OPUS studies according to KRAS and BRAF mutation status. J Clin Oncol 2010; 28(suppl): 3506.
18 Moosmann N, Fischer von Weikersthal L, Vehling-Kaiser U, et al. Final analysis of the randomized trial of the German AIO CRC study group: cetuximab plus XELIRI versus cetuximab plus XELOX as first-line treatment for patients with metastatic colorectal cancer (mCRC). J Clin Oncol 2010; 28(suppl): 3506.
19 Siena S, Tabernero J, Cunningham D, et al. Randomized phase III study of panitumumab (pmab) with FOLFOX4 compared to FOLFOX4 alone as first-line treatment (tx) for metastatic colorectal cancer (mCRC): PRIME trial analysis by epidermal growth factor receptor (EGFR) tumor staining. J Clin Oncol 2010; 28(suppl): 3566.
20 Metges J, Raoul J, Capitain O, et al. PANERB study: Panitumumab after cetuximab-based regimen failure. J Clin Oncol 2010; 28: e14000.
21 Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009; 27: 663-71.
22 ERBITUX (cetuximab) prescribing information. Princeton, NJ, Bristol-Myers Squibb; 2010.
23 VECTIBIX (panitumumab) prescribing information. housand Oaks, CA, Amgen; 2010.
24 Wilke H, Siena S, Loos A, Berghoff K, Kohne C, Van Cutsem E. Premedication and incidence of infusion-related reactions in patients with metastatic colorectal cancer treated with cetuximab plus irinotecan-based chemotherapy. J Clin Oncol 2010; 28(suppl): 3561.
25 Van Cutsem EJ, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008; 19(suppl 2): ii33-ii34.