Apr 25 2011
An article in Science Signaling by researchers at the RIKEN Research Center for Allergy and Immunology (RCAI) has clarified for the first time the mechanism governing differentiation of B cells into antibody-producing plasma cells. The finding establishes a role for the extracellular signal-regulated kinase (ERK) signaling pathway in B cell differentiation, a key step toward the development of B cell-targeted drugs for treatment of autoimmune diseases and allergies.
As the only cells in the body that produce antibodies, B cells play an essential role in the immune system's defense against bacteria and viruses. Differentiation of B cells into antibody-producing plasma cells is central to this role, initiating the production of antibodies whose targeted binding mechanism enables the immune system to identify and neutralize foreign objects. The mechanism underlying this differentiation process, however, remains unknown.
To better understand this mechanism, the research group focused on the signaling of the extracellular signal-regulated kinases (ERK), intracellular signaling molecules known to play an important role in the cell cycle and survival of immune cells. Hoping to glean insights into the role of ERKs in B cell differentiation into plasma cells, the researchers generated mice deficient in two different ERKs, ERK1 and ERK2, and studied the effect of this deficiency on the fate of B cells.
What they found confirmed that ERKs are in fact essential to B cell differentiation: B cells in mice without these key molecules were unable to form plasma cells (Figure 1). The researchers further traced this observation to a gene called Prdm1 encoding the protein BLIMP-1, increased expression of which leads to differentiation and proliferation of plasma cells in B cell immune response (Figure 2). ERKs, they discovered, phosphorylate the transcription factor Elk1, which leads to expression of Blimp-1 (Figure 3).
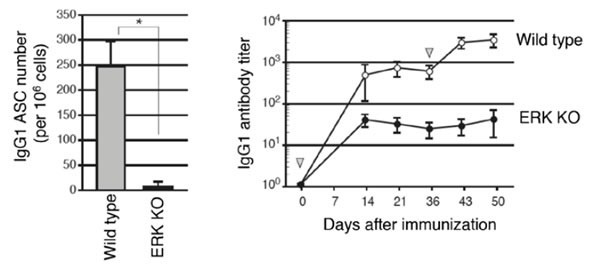
Figure 1: ERK KO mice showed impaired IgG1 antibody production
Wild type and ERK KO mice were immunized and the number of antigen-specific IgG1 antibody-secreting cells (ASC) (left) and antibody titer in sera was measured (right). Both the number of ASC and antibody titers were reduced in ERK KO mice.
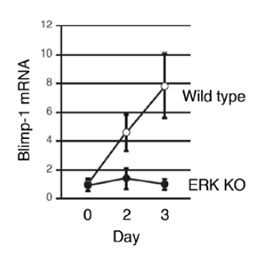
Figure 2: ERKs are essential for the induction of Blimp-1
Purified B cells were stimulated with anti-CD40 and IL-4 for the number of days indicated, and the amount of Blimp-1 mRNA was measured. Blimp-1 was induced in wild type mice, but not in ERK KO mice.
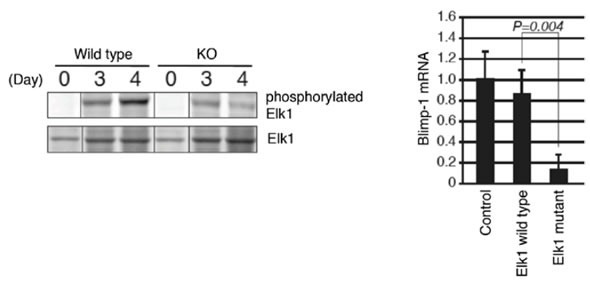
Figure 3: ERKs are required for the phosphorylation of Elk1
In vitro stimulated cells (similar to Figure 2) were subjected to Western Blotting analysis. ERK KO B cells showed impaired Elk1 phosphorylation (left). B cells with the mutant Elk1, which are genetically modified not to be phosphorylated by ERKs, showed impaired Blimp-1 induction (right).
By elucidating the role of ERKs in B cell differentiation, the current research provides valuable insight into a little-understood area of immune response, promising advances in drug discovery and offering hope to autoimmune disease and allergy sufferers around the world.
Reference: T. Yasuda, K. Kometani, N. Takahashi, Y. Imai, Y. Aiba, T. Kurosaki, ERKs induce expression of the transcriptional repressor Blimp-1 and subsequent plasma cell differentiation. Sci. Signal. 4, ra25 (2011). DOI: 10.1126/scisignal.2001592