Ultrasound devices use sound waves to image the organs of the body safely, without the use of radiation. The sound waves are produced by applying a current to a piezoelectric crystal housed in a transducer. The crystal alters shape and creates a sound wave.
When the transducer containing the crystal is applied to the patient the sound enters the body and is then reflected back to the transducer when it encounters an interface. The sound wave that is reflected back to the transducer changes the shape of the crystal and creates an electrical current. The intensity of the returning sound wave determines the intensity displayed on the image.
A gray-scale image is then created depending on the intensity of the returning echoes ranging from black, which represents fluid in the body, mid–greys which may represent organs such as the liver and uterus through to white which represents highly reflective objects such as bone.
Each organ of the body has its own characteristic pattern of echoes – disease processes change the normal echo patterns of organs enabling detection.
What are ultrasound devices used for?
Ultrasound imaging has many clinical applications including fetal monitoring, vascular imaging, procedure guidance, and general diagnostic imaging.
Handheld ultrasound systems are intended to be used as an adjunct to the physical exam where more expensive systems are not available such as:
Diagnosis. Ruling in pathology for clinical examinations of the abdomen, the pelvis, obstetric, cardiac, and the peripheral vascular system.
Screening. These types of procedures are the so-called “quick-look” examinations to rapidly determine if the patient should proceed to a further, more exhaustive ultrasound examination or perhaps another diagnostic test. A specific example of a screening tests is Abdominal Aortic Aneurysms (“AAA”) screening.
Guidance. This is one of the fastest growing applications of portable ultrasound systems. Handheld systems are suitable for large vessel vascular/venous access, pleural and abdominal fluid drainage guidance, liver biopsy guidance, and bladder aspiration.
When were handheld ultrasound devices first developed?
There is no universally accepted definition of “handheld” ultrasound devices. Some manufacturers have used the term “handheld” with ultrasound products as large as a laptop computer.
Through working extensively with clinicians Signostics developed the view that a true “handheld” should be small enough to fit in one’s pocket or to comfortably hang around a clinician’s neck, like a stethoscope, and that the device should weigh no more than one pound (450 grams).
Signostics launched the world’s first sub 1 pound handheld ultrasound in 2009 (2008 into the veterinary market).
What are the benefits of a handheld ultrasound device?
Handheld ultrasound is of greatest benefit when due to size, portability, or cost, larger and more expensive ultrasound systems are unavailable. Because of their more convenient size and substantially lower cost, handheld ultrasound devices are entering a range of clinical settings where previously ultrasound use was impractical.
The fastest growth in the ultrasound market has come from portable systems sold into “emerging markets” like emergency medicine and intensive care, and handheld ultrasound is continuing this trend.
Rural and remote doctors who previously had limited access to ultrasound departments can also assess their patients and rule-in whether they need urgent medical attention requiring travel or air lifting.
Emergency and Intensive Care physicians can assess their patients in the department saving time and improving patient management rather than waiting to transport the patient to and from the ultrasound department.
Obstetric staff are able to quickly and easily determine the presence of a baby’s heart beat and fetal position.
Hand held ultrasound devices allow simple effective ultrasound examinations to be performed at the point of care – speeding up decision making by the clinician.
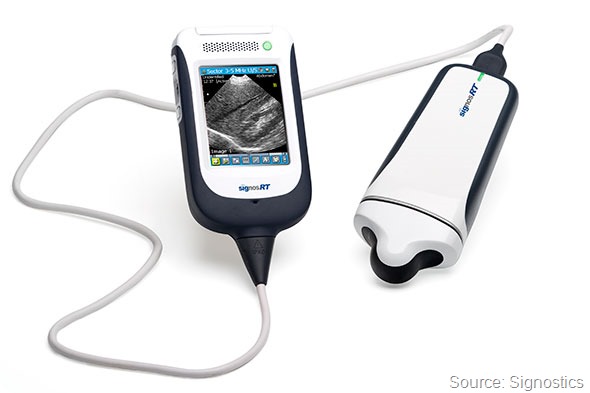
What are the main features of Signostics’ second generation portable handheld ultrasound device the Signos RT?
The Signos RT ultrasound device is a light weight, affordable, portable and an easy to use real-time ultrasound device. The transducers are interchangeable allowing versatility in the examinations performed.
The screen is a touch screen and practitioners can either navigate the tool bar with the stylus or by using the scroll wheel. B-mode, M-mode and Pulse Wave Doppler are included and multiple presets are available to optimise the image easily.
The practitioner can easily insert patient details, use the preset annotations, annotate their own text, voice record, save movies, measure dimensions and volumes, store images on the device or download to a PC or laptop using a windows system. The transducer can also be attached directly into a PC or laptop by USB if desired.
How was this device developed?
The second generation Signos RT device was developed building on the capability of the relevant aspects of the first generation device using our team of engineers. The relevant aspects remained as being hand held and affordable. To this we added real time and ease of use. Some aspects of development were outsourced to Signostics’ partners.
Our approach to the Signos RT development was to integrate user feedback on the first generation device with our medical and engineering teams working together in the development process from very early stages. This process ensured design requirements were relevant and also ensured our device is easy to use.
In the end through our validation process, we received feedback that not only is the device easy to use, but that the images seen on the device provide the user with the relevant information. This approach ensured the final product meets user needs.
How accurate is the Signos RT?
For the intended applications of the Signos RT the images are accurate and diagnostic. This device is not intended to compete with the high end ultrasound machines and the examinations they perform but to give the practitioner an accurate answer to simple questions.
As one practitioner stated through our validation process “I can obtain the same image that I get on the big machine (and) it’s easier to use.”
How does the Signos RT compare to other handheld ultrasound devices on the market?
All ultrasound machines will have their strengths and the practitioner needs to use the device that suits their patient’s needs. Some hand held devices excel at imaging the heart while the SignosRT is a good all round general purpose device capable performing a variety of examinations for a variety of practitioners.
The resolution of the image is similar or slightly better than other devices depending on the examination performed with the added versatility of being able to attach the transducer into a PC.
The interchangeable transducers make the SignosRT more versatile than other devices. The SignosRT is very user friendly and intuitive to use and more affordable than other devices.
Where is the Signos RT currently available?
Signos RT is currently available throughout most of the major ultrasound markets in the world including Australia, New Zealand, Europe, parts of Asia and for the veterinary market in the USA under the brand name of Sonimage P3, distributed by Konica Minolta Inc.
If anyone would like to buy our device, they can contact us through our website.
Availability in the USA human market is still subject to FDA approval - we have submitted our 510(k) application and the FDA are assessing it.
How will Signostics be distributing the Signos RT device?
Signostics we will be utilising our network of very effective Distributors including Konica Minolta.
What impact do you think the Signos RT will have on medicine?
We envisage that in the coming years, handheld ultrasound products will become as ubiquitous as the stethoscope. Physicians around the world will have in their pocket the capability of a Signos RT to look inside the patient’s body.
We expect the trend of requiring point of care ultrasound though the Signos RT to continue – improving patient care, reducing costs, and increasing the efficiency of physicians.
What are Signostics’ plans for the future?
Our future is to become a major player in the medical device industry. We are able to utilise a great team of people and be very quick and nimble in commercialising new products even though we operate in a highly regulated market.
Signostics has more than just imagination and has a road map of future products that we intend rolling out in due course. These will build on the Signos RT platform and deliver a range of improved solutions to clinicians.
Where can readers find more information?
At our website – www.signostics.com.au or by email to [email protected] .
About Warren Ortmann
 Warren Ortmann was appointed Chief Executive Officer of Signostics in October 2012 and was previously Chief Financial Officer of the Company.
Warren Ortmann was appointed Chief Executive Officer of Signostics in October 2012 and was previously Chief Financial Officer of the Company.
Warren has accumulated nearly 25 years experience in accounting roles, is a CPA with the Australian Society of Certified Practising Accountants and holds an MBA from the University of South Australia.
Warren spent 21 years with Sola Optical / Carl Zeiss Vision an Ophthalmic Lens supplier in various capacities, ending up as the CFO for the Asia Pacific Region, the last 3 years of which whilst living in Singapore.
Warren brings extensive Asian and commercial manufacturing experience to Signostics and also the experience of completing several M&A transactions.
Previous CFO positions include a time at an Automotive Tier 1 and 2 manufacturer and a home based nursing organisation.