May 23 2013
Observing surfers with cystic fibrosis (CF) led scientists to discover that the inhaled mist of seawater has a therapeutic effect on the lung problems associated with the disease. Now the findings have been used by pharmaceutical company Parion Sciences and product development firm Cambridge Consultants in a revolutionary new aerosol delivery system. It enables CF sufferers to get the benefits of saltwater treatment in their own homes overnight while they sleep.
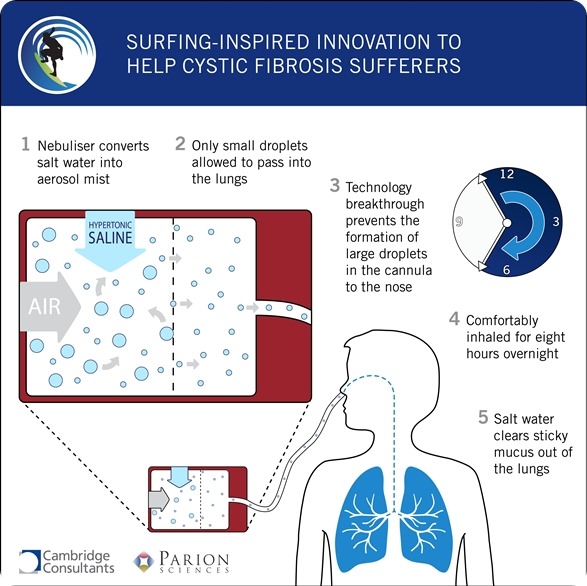
“We have seen evidence that routine exposure to salt water reduces the number of lung infections in CF patients and leads to fewer hospital admissions – but the challenge was to develop an optimal delivery system,” said Dr. Richard Boucher, co-founder and chairman of Parion. “We enlisted Cambridge Consultants to design the system, given its track record of creating world firsts in drug delivery device development – and the results have exceeded our expectations.”
CF is a chronic disease that affects the lungs and digestive system of more than 30,000 adults and children in the US and more than 70,000 worldwide. Studies have shown that the inhalation of a hypertonic saltwater solution improves the condition of sufferers by rehydrating the layer of mucus film that lines their lungs. Cambridge Consultants conducted human-factor studies and applied its expertise in fluid dynamics to develop a system that could deliver an aerosol mist through the nose continuously for eight hours.
“We immediately recognized the potential of this project to transform the lives of CF patients,” said Matthew Allen, program director at Cambridge Consultants. “The challenge was to build an aerosol nebulizer system that could be comfortably used by patients overnight – with the saline mist travelling down a long cannula to the sleeping patient without forming the large droplets that often occur in a standard nebulizer system. The size of the saline droplets is crucial to the success of the treatment as they need to be small enough to penetrate deep into the lungs.”
The tPAD (trans-nasal pulmonary aerosol delivery) device has been assessed in a clinical trial – and so greatly exceeded its predicted performance that additional devices have now been requested to support further clinical trials. The aim is to provide an easy and effective treatment system for CF patients that is suitable even for young children and allows the disease to be treated at the earliest possible stages.
Source: www.CambridgeConsultants.com