A group of Australian researchers, back in 2006, noted that there was a link between the severity of cystic fibrosis between sufferers who surfed, and sufferers who did not surf – and obviously Australia is a good place to spot that.
So it was a very anecdotal observation. It was purely that, if you surf and you suffer from cystic fibrosis, you have less severe symptoms than if you didn't.
That obviously then led the Australian research groups to investigate why that might be.
The company that we’ve been working with, which is a US company called Parion, were founded by respiratory medicine academics who were interested in this phenomenon.
They picked up on the research in Australia and continued their own research into what might be the physiological effect that these people were observing anecdotally.
By what mechanism is the inhalation of a saltwater solution known to improve CF patients’ symptoms?
So cystic fibrosis is a genetic disease. The genes that regulate the way which the cells in the lungs generate mucous don't work properly, so cystic fibrosis patients cannot remove mucous from their lungs. Mucous builds up in their lungs.
That has a number of effects, one of which is that cystic fibrosis sufferers are unable to get rid of lung diseases that the rest of us deal with by this kind of mucosal removal action.
The reason the mucous builds up in the lungs, or can't be removed from the lungs, is to do with a rather complex mechanism of ion transfer.
The chloride ions and sodium ions can't be transferred through the cells in the lungs. So Parion investigated the effect of ions on these cells by actually growing cells in a culture, exposing them to salt solutions, and observing the way they work.
They observed that you could make the cells function, kind of normally, if there was the presence of sodium chloride solution, saline solution, on the cell. They could get this transfer of ions which regulates the normal transport of mucous
Parion managed to build a model that demonstrated how this mechanism works. Why the presence of saline in your lungs can help this mucosal transfer.
Having established that there was a physiological reason why the presence of saline in the lungs can improve mucosal transfer; the next problem is how do you expose the patient to this saline in their lungs.
There is research going on in a number of different centers, but one of the observations is that if you provide saline to the lungs of cystic fibrosis patients, this mucosal transfer mechanism will work; when take away that saline, the mucosal transfer mechanism breaks down again. It only works so long as you are continuing to provide the saline.
What Parion determined was that if you could provide the saline over a long period of time, not only did you provide relief for longer, the effect lingered for longer after the saline treatment was removed.
If you expose the patient to this saline aerosol for a few minutes, as soon as you turn it off, the patient reverts back to normal very quickly.
If you expose them to saline for a long time, when you turn it off, their lungs continue to operate as normal. Eventually, obviously it will revert back to the original state.
The challenge that was set to Cambridge Consultants was to develop a device that was able to deliver a saline aerosol to a patient for a long period of time.
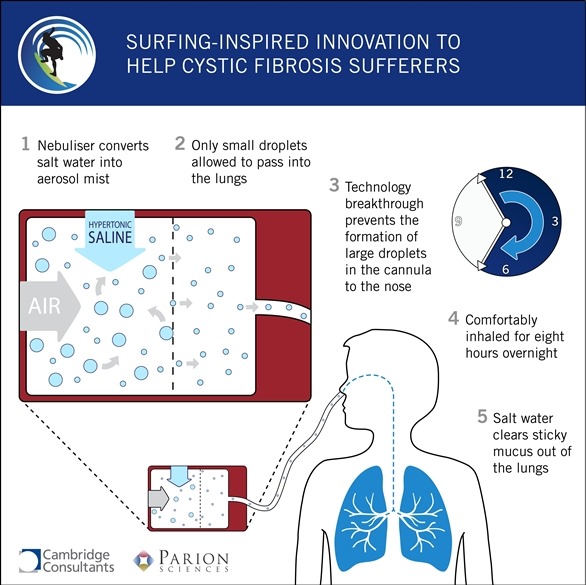
How did Cambridge Consultants aid Parion Sciences to develop a saltwater treatment device for CF patients?
The technique to create an aerosol of a saline solution is relatively simply. There's a lot of existing technology that can do that.
The challenge is to create an aerosol that can be delivered to the patient very comfortably, because the patient is breathing this aerosol while they are asleep.
The answer is to deliver the aerosol through the patient's nose so there's nothing covering their face. There's just a cannula in their nose, it's quite a comfortable arrangement.
The cannula itself, the tube, needs to be quite a small tube to be comfortable. If you try and get a liquid aerosol to travel through a long tube it tends to condense in the tube, form droplets which block the tube or drip, which is uncomfortable, waking you up and even making you choke. The challenge was not to get any drops in the tube.
What Cambridge Consultants did was develop a technique for creating an aerosol that would not condense in the tube, therefore the patient always receives a very fine mist - there are never any drips. That was the main thing that Cambridge Consultants were doing.
We were engaged at the stage where there was no clinical evidence in a patient that this treatment would be effective. It was all done on the bench. It was early stage. We were developing prototypes that would enable Parion to carry out clinical trials of this technique.
The simple way of describing the way we did it was we created an aerosol of saline, of saltwater, and selectively removed all of the larger droplets. Only allowed the smaller droplets to leave the device.
It's a large droplet capture technology, and is the subject of a patent application.
How long did this development take and what did the process involve?
We first engaged with Parion about two years ago. The program has been both experimental and theoretical.
We built computational fluid dynamic models which model the aerosol and the equipment that it had to go through. At the same time, we built prototypes to test our different designs that were created through this computational route.
We also used some sophisticated droplet size measurement techniques;laser diffraction measurement of the droplet size that was coming out of this device.
But not only did we concern ourselves with the technology around getting this perfect aerosol, we also carried out user studies with the user groups to ensure that the device itself would be comfortable and appropriate for you to use whilst you were asleep.
So we had to assess the comfort of the cannula in the patient's nose, the noise that the device would make - the actual environment that you would use it in. We had to make sure that it was appropriate for using by your bed side and that it would fit in with people's lives. . We consider the whole usability aspect of the device, as well as the technology.
What hurdles had to be overcome in the product development process?
I think the major hurdles are the existing technology for creating aerosols for medical treatments. Basically, nebulizers are generally designed for relatively short treatments of a few minutes and generally used through the patient’s mouth using a face mask or a tube.
We had to take those sorts of technologies and process the aerosol they produced for really quite a different application to the patient.
Instead of looking at ways to engineer an aerosol cloud that we wanted, we had to post engineer an aerosol cloud that we didn't want to make it the right sort of aerosol. Really the challenge for us was to use as far as possible pre-existing technology to produce an aerosol, and then create a novel approach to process it . That's not very commonly done.
At what stage of development is the aerosol system currently at?
An early phase clinical trial has been carried out. That trial was looking at the distribution of droplets in a patient's lung.
Healthy patients, not cystic fibrosis patients, inhaled a solution which has a radioactive marker in the solution so that the patient could be imaged. It's called scintigraphy. The patient can be imaged, and you can observe the distribution of the droplets in the lung.
That study has been completed and we observed the device to be significantly more efficient than we expected it to be, which was a great result.
We are currently building a series of improved prototypes for the next phase of clinical trials. The device is currently in a series of clinical trials with different patient groups, including children, and it parallels the commercial development of the product, Parion are commercializing the product.
What impact do you think this product will have on the lives of CF patients?
Cystic fibrosis patients, depending on the stage of their disease, have to take a lot of treatments, a lot of antibiotics for instance, to counteract the fact that they don't have this mucosal clearance and are prone to lung diseases.
This treatment means that during the eight hours whilst they're asleep, they should have relatively normal mucosal behaviour and even when they wake up, that should continue through the day. It should reduce their reliance on the drugs that they have to take to treat the infections that they would otherwise get.
Now those treatments tend to be time consuming. There are other nebulized solutions that they use to get the drugs into their lungs. There are physiotherapy treatments that they have to go through. All of those should be reduced by the fact that they had this long term treatment.
It's a quality of life issue. It's a genetic disease. It's a chronic disease. So this treatment doesn't cure cystic fibrosis, it simply prevents all of the side effects of cystic fibrosis, which I'm afraid are eventually fatal. It doesn't necessarily prolong life, but it does improve the quality of life.
Are there any plans in place to develop additional devices?
Yes. Parion is a start-up company, an entrepreneurial start-up company. This technology could be used to deliver other drugs, for treatment of diseases like asthma, or COPD, other lung diseases.
I believe that Parion have an ambition to pursue other treatments for other lung diseases but right now cystic fibrosis is their main aim, but it's a platform technology.
What are Cambridge Consultants plans for the future?
We remain engaged with Parion. We're looking at improvements to the device to improve efficiency.As I said earlier on, this is still an early stage clinical product. For it to be a commercial product there would have to be a number of engineering improvements, optimizations. We hope to remain engaged with Parion, so long as they need us.
The commercial exploitation, there's a number of different routes that Parion could take. We remain in support, but clinical trials take some time, so it's going to be a long road.
Where can readers find more information?
About Matthew Allen
 Matthew Allen is a Program Director at Cambridge Consultants. He is responsible for the company's drug delivery business which specializes in the development of novel devices on behalf of pharma and device companies.
Matthew Allen is a Program Director at Cambridge Consultants. He is responsible for the company's drug delivery business which specializes in the development of novel devices on behalf of pharma and device companies.
Matthew has a PhD in Surface Science from the University of Liverpool and has over 15 years' experience developing breakthrough medical devices in respiratory medicine.