
Please can you outline how cervical cancer is currently diagnosed?
It's a two-step process. In developed countries, women are invited for a smear test which mainly uses LBC (liquid-based cytology), where a sample of cells are taken from the cervix and sent to a cytopathology lab for analysis.
The result of that test may be normal, in which case the patient is told to return for another test in 3 to 5 years, or it may be abnormal and the patient is referred to a colposcopy clinic for further examination.
At the colposcopy clinic, the clinician will examine the cervix using a magnification tool called a colposcope to assess the extent of any abnormalities. This involves applying a 5% acetic acid onto the cervix and a clinician views uses a colposcope, which is a magnification tool to visualise and checking for areas of white discoloration, which has some correlation with the disease.
However, the clinical decisions made at this stage are somewhat subjective and based on an assessment of what the clinician can see – a clinician’s impression. If the tissue appears severely abnormal, a biopsy is taken and sent for histopathological analysis which will confirm whether disease is present or not.
There are then two treatment options, depending on the stage of disease. If the tissue is pre-cancerous a large part of the cervix called the transformation zone is excised.
In the case of cancer, the patient is offered a wider range of treatments including surgery, radiotherapy or chemotherapy, depending on the extent of the cancerous lesions and the cancer spread.
In what ways is this diagnostic process limited and what sparked Zilico to develop a cervical cancer diagnostic system?
About 10 years ago, two professors just happened to meet while they were travelling by train and began discussing the limitations of current methods for cervical cancer diagnosis. One of the professors has a background in medical physics and the other a background in gynecology and their discussions led to the development of the technology behind our product ZedScan.
The first trial was conducted in 2000 and was published in the Lancet, which showed the potential of this technique for reducing the subjectivity I described. The other advantage is having the results in real-time.
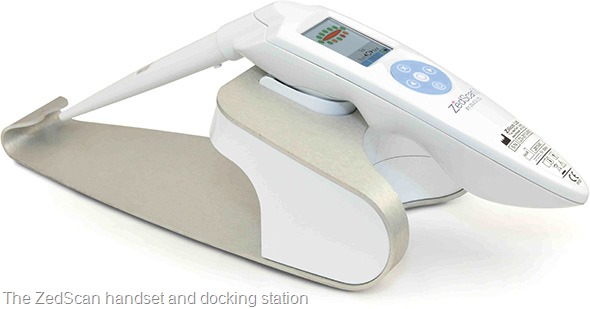
Please can you give a brief introduction to ZedScan and how it works?
Electrical impedance spectroscopy (EIS) provides a means of examining tissue structure, something scientists and pathologists are already doing all the time, except they do it from a visual perspective
EIS analyses tissue structure by measuring the electrical properties of cells and observing the changes in conductivity of the tissue as the structure changes – from a healthy state to early and then late pre-cancer and finally, to a cancerous state.
The results of this tissue analysis is interpreted using an algorithm to provide a diagnosis for the clinician. This enables the clinician to better manage a patient at the first visit.
ZedScan is said to offer more accurate detection of cervical neoplasia in real-time, please can you explain how this is achieved?
We are moving away from the subjective nature of current methods. Our technology provides a template that works exactly the same way every time.
In addition, the device interrogates the tissue to a depth of about 400 microns, compared with the current optical techniques which only enable the clinician to view the surface of the cervix.
Obviously, there is still the risk of human error, which will vary between those operating the device, but with the right training, each device will assess the cervical tissue in exactly the same way if repeated 100 times and results are therefore highly reproducible.
The trials we have carried out, five in total, which have demonstrated the efficacy of the device and shown that it is more accurate than what is out there today.
What are the main benefits of real-time detection?
ZedScan provides the clinician with more detailed and accurate information on the status of the cervix which is immediately available as soon as the examination has been completed. This then enables them to make a more informed decision about how to proceed at the patient’s first visit.
That decision may be to treat a patient immediately, it may be to take a directed biopsy using the device, or if no disease is detected, they may return the woman back to routine surveillance. The clinician knows that there are three routes that can be taken and provides them with the confidence to determine which one of these can be taken today.
The “See and Treat” approach also offers advantages to the patient. If a woman does happen to have a high-grade abnormality, she can be treated there and then rather than return for treatment at a later date. That not only benefits the healthcare system, but also has a positive socioeconomic impact, in that a woman doesn't have to take more time off work or organize additional childcare, for example all those social issues come into play. The real-time aspect really can benefit both the clinician and the patient.
Anxiety is another factor to consider. A number of studies have shown that compliance with cervical screening is an issue. A certain, small proportion of women do not attend their screening appointments and anxiety is thought to play a part.
We believe that if you can shorten the time that a women needs to spend in hospital, the frequency of the visits required, then collectively the different factors will have a beneficial impact.
We don't have evidence to support this yet, and we don't push these points from a marketing angle, but our market intelligence plus our intuition tells us this may be the case.

Please can you outline the clinical trials that have supported the clinical efficacy of ZedScan?
Five clinical studies have been done to date, all of which have been published in peer-reviewed journals. The last one was pivotal for us in terms of regulatory approval and that was published last year in the British Journal of Obstetrics and Gynecology. This trial was carried out across three hospitals in the UK and Ireland and recruited 429 patients in total.
Are there any limitations of ZedScan?
There is a problem with regard to auditing disease in its entirety. Sometimes, the cervix of some women can be introverted and it becomes difficult to see the junction where the two tissues of the cervix come together. This is a recognized limitation of all current techniques and in this respect; it is not the panacea for cervical cancer diagnosis.
ZedScan is currently used alongside colposcopy on women identified with abnormal smear results. Do you think ZedScan will one day replace the need for colposcopy or will it always be used as a complementary system?
ZedScan would not replace colposcopy but would be used in conjunction with it. The purpose of screening is to identify and treat women with disease. We don't provide a treatment option with our devices, which are purely diagnostic tools.
The excision that I mentioned still needs to be performed by a clinician requiring a magnified view of the cervix. Colposcopy will always be needed, but perhaps the emphasis on why it is needed will change, essentially becoming a way of magnifying the cervix, while our system provides the accurate diagnosis.
We are developing a sister product of ZedScan which we believe may be useful for front-line screening but we've yet to deliver the clinical evidence for it, which is part of our plan as our company grows.
ZedScan has recently received CE certification. What impact will this have?
The CE mark gives us the regulatory approval to market and sell this product as a diagnostic system and, more importantly, enables clinicians to feel they can use this device in normal clinics.
From a company perspective, the CE mark essentially opens ZedScan up to the European market and allows us to sell it for use in live clinical settings anywhere in Europe.
In addition, the mark lowers the regulatory hurdle in countries where the CE mark is not directly applicable. The authorities in those countries can at least be assured by the fact that ZedScan has the CE mark, which reduces the regulatory burden for us to bring the product to market in China or Australia for example, where the regulatory systems are different.
What excites you most about ZedScan?
We have acquired significant clinical evidence from trial settings but now we are generating more anecdotal evidence, from commissions across the globe.
For example, in the hospital where ZedScan is currently being used, we know that one clinician's initial impression on visual examination of a woman's cervix was that the pre-cancer was low-grade, while the device suggested it was high-grade. When the biopsy results were returned, the pre-cancer was indeed high-grade and consequently the woman was treated., This patient would potentially have been mis-diagnosed and treatment delayed if this system had not been in place.
Hopefully, ZedScan is making at least a small difference in the diagnosis and management of cervical disease and the patients who have it.
What are Zilico’s plans for the future?
In the short-term, the plan is to drive the adoption of ZedScan in the marketplace and in turn the commercial sales
In the medium-term, there are a couple of other exciting applications such as the screening potential of the sister device to ZedScan that I mentioned. We have also done some early trial work on its application in oral cancer. I am very pleased with the initial data and we are starting to take those developments forwards.
In the longer-term, we've got some other exciting potential products in the pipeline. One example is an intra-operative device that a clinician can use to look for diseased abnormal tissue rather than having to look visually, which is again subjective.
We talked to an ENT (ear, nose and throat) specialist who described how helpful real-time information would be to him while performing surgery. We absolutely do have exciting plans for the future.
Where can readers find more information?
http://www.zilico.co.uk/
About Sameer Kothari
 Sameer brings over 15 years commercial experience to Zilico. Prior to joining in November 2007 Sameer was CEO of Plasso Technology Ltd, a venture capital-backed company developing research tools and labware for the life sciences. 2007 saw the launch of its first product, EpranEx, a new generation research tool for life scientists.
Sameer brings over 15 years commercial experience to Zilico. Prior to joining in November 2007 Sameer was CEO of Plasso Technology Ltd, a venture capital-backed company developing research tools and labware for the life sciences. 2007 saw the launch of its first product, EpranEx, a new generation research tool for life scientists.
In May 2007, Sameer successfully led a trade sale exit of Plasso to Becton Dickinson Inc. Prior to joining Plasso, Sameer was a senior executive, with roles in strategy, business development, HR and M&A within Marconi plc. Including a management board position within mainland Europe.
Sameer is a fellow of the Winston Churchill Trust and The British American Project and a Trustee of a not-for-profit organization. Sameer is a keen sailor having sailed in the BT Global Challenge in 2001 from Sydney to Southampton.