Sep 4 2014
New data from the international FIELD Study Repeat presented at the 15th World Congress of Cancers of the Skin (WCCS) in Edinburgh, Scotland, demonstrate initial treatment with Picato® (ingenol mebutate gel) 0.015% is efficacious in treating actinic keratoses (AK) and, when followed by a repeat cycle for persistent and newly emerging AKs, this efficacy is further increased.1 The data also confirm two treatment cycles of Picato® are well tolerated by patients.2
These findings are significant as AK is a chronic disease.1 The presence of underlying sub-clinical (invisible) lesions can lead to recurrence of original AKs and the development of new lesions over time3, which normally require repeat treatment. The FIELD Study Repeat followed patients over a one-year-period, confirming both the short and in particular the long-term benefits when treating once or twice of Picato® – with an overall 50% complete clearance of all AK lesions after one year*.1
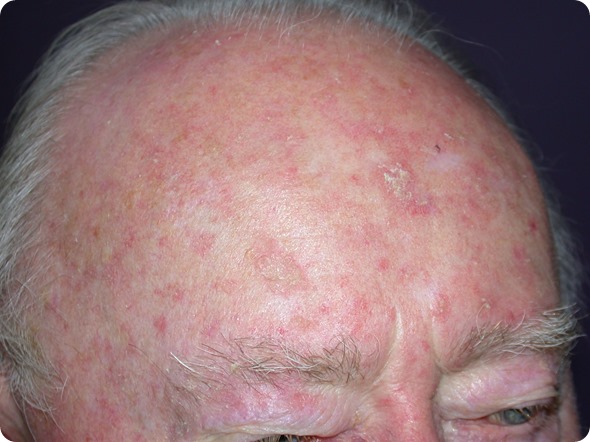
Actinic keratosis on the scalp of a male
Presenting the data at WCCS today, Professor Claus Garbe, Department of Dermatology, University Hospital Tübingen, Germany and Principal Investigator of the FIELD Repeat Study, commented:
“AK is a very common precursor to non-melanoma skin cancer, which requires ongoing treatment. The new data from the FIELD Study Repeat validate Picato® as a well-tolerated field-directed therapy, suitable for the long-term treatment of actinic keratoses, showing an overall high complete clearance rate after one year.”
The FIELD Study Repeat is part of the larger international FIELD Study Programme being run by LEO Pharma, designed in partnership with leading clinicians to investigate the potential broader role of Picato® in the management of AK.
Additional data from the FIELD Study Programme was recently published in the Journal of Drugs in Dermatology (JDD)4 with the long-term results of the FIELD Study 1, investigating the efficacy of Picato® as a field-directed therapy used sequentially following treatment with cryosurgery.4
Results show that combining lesion-specific cryosurgery with Picato® field therapy significantly improves the clearance of AK lesions, with an overall 30.5% complete clearance rate, compared to 18.5% with cryosurgery plus vehicle gel over a one year period.4
This elevated efficacy results from enhancing the effect of cryosurgery with Picato® on the visible baseline lesions, and from a ‘field treatment effect’ of Picato® on subclinical lesions not visible at baseline (demonstrated by fewer patients experiencing new lesions when treated with Picato® compared to vehicle gel).4 The data also confirm Picato® field therapy after cryosurgery is well tolerated.4
Dr William Hanke, Founder and Medical Director, Dermatologic Surgery, Laser and Skin Center of Indiana, US, and FIELD Study 1 Principal investigator, added:
“The recently published 12 month data from FIELD Study 1 show sustained patient benefits of field treatment with Picato® in combination with cryosurgery. These data are now reinforced with evidence that repeat use of Picato® is efficacious and well tolerated over the same time period. Combined, these studies provide further evidence for the role of field treatment with Picato® in achieving long-term clearance of AK lesions.”
Follow @LEOHealthySkin for the latest updates from #WCCS2014.
*Patients treated twice with Picato® or treated once and remained clear at 8w, 26w and 44w, 50% were still clear at 12m (44.0%-56.1%, 95% CI)
References
- Garbe C. Ingenol mebutate gel 0.015% repeat use for multiple actinic keratoses on face and scalp: a 12 month pahse 3 clinical study. Presented at the World Congress on Cancers of the Skin, Edinburgh, Scotland. 4 September 2014
- LP0041-22 Clinical Study Report EudraCT no. 2011-005018-13 01-Jul-2014 page 1,165
- Berman B et al. Cutis. 2012; 89:241-50
- Berman B et al. Journal of drugs in dermatology : JDD. 2014; 13:741-7
- Lebwohl M et al. N Eng J Med. 2012; 366:1010-9
- Goldberg L and Mamelak A. Journal of drugs in Dermatology. 2010; 9:1125-32
- Frost C et al. The British journal of dermatology. 1994; 142:722-6
- Sober A. Cancer. 1995; 75(2 Suppl):645-50
- Ulrich M et al. Expert Opinion. 2010; 15:545 - 55