Sep 19 2014
Neuromod Devices (www.neuromoddevices.com), the Irish medical devices company, today announced that it has successfully secured ISO 13485 certification of its Quality Management System.
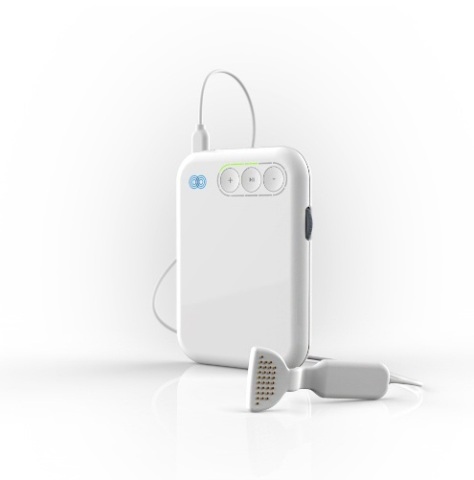
The company, which is headquartered at NovaUCD, the Centre for New Ventures and Entrepreneurs, has secured this certification ahead of the launch of its multisensory tinnitus treatment mutebutton™, scheduled for December.
According to Dr Ross O’Neill, CEO, Neuromod Devices:
This is an important milestone for the company. This certification independently validates our ability to deliver medical devices and services while consistently meeting both customer and regulatory requirements. It is a testament to the quality of work ongoing within the company ahead of the company’s first product launch later this year.
Tinnitus is estimated to affect 10% of the population with 1% suffering from severely disabling effects. At present there are limited avenues of treatment for subjective tinnitus sufferers and medical professionals alike.
The mutebutton™ system has been developed to address this need and combines synchronous audio and lingual (tongue) stimulation to promote patient neuroplasticity, the manipulation of the brain’s ability to learn and re-learn. Used for a minimum of 30 minutes a day, over a 10-week period, the treatment has been shown to gradually reduce the sounds of tinnitus in clinical studies conducted at NUI Maynooth and at the Hermitage Medical Centre in Dublin.
“The global study of neuromodulation continues to unlock new and exciting surgical and non-surgical treatments for patients. Neuromod Devices is committed to unlocking the potential applications of this exciting technology while meeting the highest standards for our regulators and customers alike,” added Dr O’Neill.
The company anticipates the Irish launch of the mutebutton™ system this December, ahead of a wider European launch planned for 2015.
Source: http://www.neuromoddevices.com/