Dec 19 2014
Somatuline® is the first and only antitumor therapy demonstrating a statistically significant progression-free survival benefit in a combined population of patients with gastrointestinal and pancreatic tumors
Ipsen Biopharmaceuticals, Inc., an affiliate of Ipsen (Euronext: IPN; ADR: IPSEY), today announced that Somatuline® Depot® (lanreotide) Injection 120 mg (referred to as Somatuline®) was approved by the U.S. Food and Drug Administration (FDA) for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in adult patients with unresectable, well or moderately differentiated, locally advanced or metastatic disease to improve progression-free survival (PFS).
 “The approval of Somatuline® for the treatment of patients living with gastrointestinal and pancreatic tumors is a major achievement for Ipsen that will provide a new therapeutic option with the potential to help many of the thousands of patients in the United States suffering from this devastating disease,” said Cynthia Schwalm, President and CEO of Ipsen Biopharmaceuticals, Inc. “Somatuline® is the first and only treatment with a statistically significant progression-free survival benefit approved by the FDA for patients as an antitumor therapy in the treatment of gastrointestinal and pancreatic neuroendocrine tumors. This is a significant step forward in our mission to develop and deliver innovative therapies to treat serious illnesses.”
“The approval of Somatuline® for the treatment of patients living with gastrointestinal and pancreatic tumors is a major achievement for Ipsen that will provide a new therapeutic option with the potential to help many of the thousands of patients in the United States suffering from this devastating disease,” said Cynthia Schwalm, President and CEO of Ipsen Biopharmaceuticals, Inc. “Somatuline® is the first and only treatment with a statistically significant progression-free survival benefit approved by the FDA for patients as an antitumor therapy in the treatment of gastrointestinal and pancreatic neuroendocrine tumors. This is a significant step forward in our mission to develop and deliver innovative therapies to treat serious illnesses.”
The approval of Somatuline® was based on a 96-week landmark registrational Phase III, double-blind, placebo-controlled study (CLARINET®) of 204 patients enrolled in 48 centers across 14 countries. The trial showed that Somatuline® reduced the risk of disease progression or death by 53% versus placebo in patients with advanced gastrointestinal and pancreatic neuroendocrine tumors (p<0.001). Safety data generated from the Phase III study were consistent with the known safety profile of Somatuline®. The rates of discontinuation due to treatment-emergent adverse reactions were 5% (5/101 patients) in the Somatuline® arm and 3% (3/103 patients) in the placebo arm.
Gastrointestinal and pancreatic neuroendocrine tumors are rare, but serious cancers. There are an estimated 112,000 individuals currently living with neuroendocrine tumors in the U.S., and the incidence and prevalence of this type of cancer have risen 4-to-6 fold in the last 30 years. Furthermore, up to ninety percent of patients are incorrectly diagnosed, many for more than 5 years, meaning that they are often diagnosed at a late stage. During this process, patients may be misdiagnosed with other gastrointestinal diseases, such as irritable bowel syndrome or Crohn’s disease.
“Somatuline® is the first somatostatin analog to demonstrate a statistically significant improvement in progression-free survival, a clinically significant endpoint in oncology which measures how long the patient continues to live with the disease without it getting any worse,” said Dr. Alexandria Phan, Director of GI Medical Oncology at Houston Methodist. “Somatuline® offers a new weapon in our fight against this deadly disease.”
Maryann Wahmann, President of the Neuroendocrine Cancer Awareness Network and a Carcinoid Cancer Patient added:
As a patient who also started an advocacy group for this community, I know first-hand how important it is to have a new treatment option with anticancer benefits for neuroendocrine tumors.
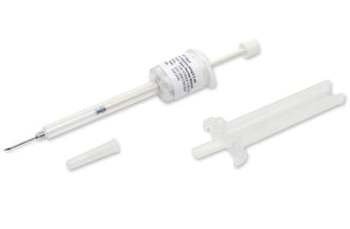
Somatuline® will be delivered via a newly approved, ready-to-use, prefilled syringe which incorporates Safe’n’Sound® technology, including a retractable needle guard to help avoid needle sticks, and it is manufactured without latex or natural dry rubber. The new delivery device does not require reconstitution and is a low volume (0.5 mL) deep subcutaneous injection offering a streamlined process that supports full dose delivery.