EKF Diagnostics, the global diagnostics company, announces that it will be highlighting the robustness of its new DiaSpect Tm point of care (POC) hemoglobin analyzer at Arab Health 2015, 26-29th January, Dubai, UAE. Also on Stand Z1G30, EKF will be discussing its new liquid-stable assay* for early sepsis detection, as well as previewing SensPoint, a new hand-held lactate analyzer with built-in connectivity functions to hospital or laboratory information systems.
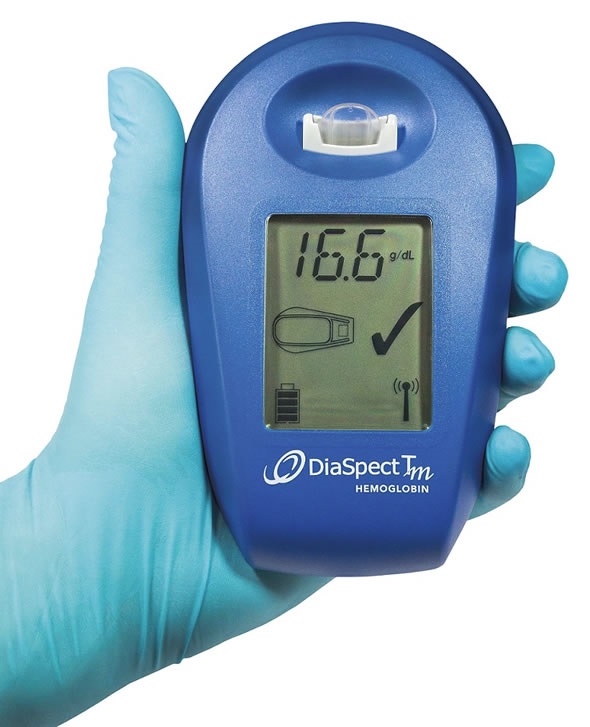
Image credit: EKF Diagnostics
Rapid hemoglobin results in all climatic conditions
EKF’s new DiaSpect Tm lightweight, palm-sized hemoglobin analyzer is fast, easy to use and reliable for anemia diagnosis in all climatic conditions. By combining laboratory quality performance with unmatched measurement speed and extensive battery life of 40 days of continuous use, or 10,000 tests, this makes it an ideal POCT device.
DiaSpect Tm is already proving highly effective in challenging POCT conditions in many locations, including east Africa and south-east Asia. In such environments heat and humidity can affect the performance of a POC device and, more importantly, the cuvettes used for blood collection. Crucially, DiaSpect Tm is unaffected by these environmental factors due to its reagent-free disposable cuvettes, and the fact that it has no moving parts. DiaSpect Tm’s extremely long battery life, also ensures this handheld device is ideal for use outside of laboratories, or in resource poor locations.
The plexiglass microcuvettes used by DiaSpect Tm have a shelf life of 2.5 years and can be stored from 0 to 50°C, meaning that refrigeration is not necessary - short term storage is possible at -30°C to 70°C for a maximum of 24 hours. Also making it ideal for POC use in all locations, DiaSpect Tm is factory calibrated against the HiCN reference method in accordance with ICSH and requires no re-calibration or maintenance, as it undertakes an automatic self-check between every measurement.
DiaSpect Tm is easy to use and requires minimal training as the user simply collects a capillary or venous blood sample of <10 µL in the microcuvette and inserts it directly into the analyzer. Hemoglobin results, which be downloaded to a PC via USB or Bluetooth, are delivered in one second.
Early sepsis diagnosis
The new CE marked Liquicolor® Procalcitonin liquid-stable assay* can be used on most automatic chemistry analyzers as an early and specific indicator of sepsis. Since Procalcitonin (PCT) levels elevate during systemic bacterial infection and sepsis, PCT can be used to quickly assess initial severity of sepsis.
The new Liquicolor® PCT immunoturbidimetric assay is precise, convenient and cost effective as it is available in a liquid-stable format, which can remain stable on-board a clinical chemistry analyzer for up to four weeks, and requires a minimal sample of just 20 µL. This assay can also improve laboratory workflow as it can be run on a primary chemistry analyzer and eliminate the need to split a sample. The reagent kit, calibrator and control sets are all available separately.
For more information on Procalcitonin, the DiaSpect Tm hemoglobin analyzer or EKF Diagnostics, please visit www.ekfdiagnostics.com.
* The Liquicolor® Procalcitonin liquid-stable assay is unavailable for sale in USA, France, Germany, Austria, Spain, Italy and Japan
About EKF Diagnostics
www.ekfdiagnostics.com
EKF Diagnostics Holdings plc, which includes the EKF Diagnostics, EKF Molecular, Stanbio Laboratory, Separation Technology Inc., DiaSpect and Selah Genomics brands, specializes in the development, production and worldwide distribution of point-of-care blood analyzers for use in the detection and management of diabetes, anemia, lactate and kidney related diseases. Its new molecular division, EKF Molecular Diagnostics, focuses on technology used within the development of companion diagnostics, specifically within oncology. EKF products are sold in more than 100 countries around the globe, through a network of specialist distributors.
Point-of-care diagnostics: EKF Diagnostics designs and manufactures world-class diagnostic devices, as well as distributing rapid test kits for infectious diseases and pregnancy. The EKF analyzer range is used widely in GP surgeries, pharmacies, blood banks, sports clinics, hospitals and laboratories for glucose, lactate, hemoglobin, hematocrit and HbA1c measurement.
Central laboratory: EKF, through its wholly owned subsidiary, Stanbio Laboratory (Boerne, Texas, USA), manufactures a comprehensive range of clinical chemistry reagents. In addition, EKF Life Sciences (Elkhart, Indiana, USA) manufactures enzymes used in reagent development and also provides contract fermentation facilities.
Molecular Diagnostics: In March 2013 EKF set up a new division to focus on molecular and companion diagnostics following the acquisition of UK-based 360 Genomics. EKF Molecular Diagnostics’ technology, PointMan™, is capable of detecting mutant genes from tiny biopsy and blood samples and has recently entered a partnership with the world renowned cancer research center at Massachusetts General Hospital, USA.
EKF Diagnostics’ strengths lie in its multi-national research and manufacturing facilities, teams of experienced analysts and engineers in Germany, Ireland, USA and the UK, and a board led by some of world’s foremost authorities in medical diagnostics.