Historic five-year results from the world’s largest clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) were presented last week at the Leipzig Interventional Course, in Germany. The data confirm long-term patency for patients treated with Zilver® PTX®.
Michael Dake*, M.D., of Stanford University, told attendees that:
five-year data for Zilver PTX versus bare-metal stenting confirm a sustained benefit for the paclitaxel-eluting stent and continues to show benefit through five years with no late catch-up. In fact, we see the benefit of Zilver PTX is even greater over time when compared to standard care.
The Zilver PTX Randomized Trial of Paclitaxel-Eluting Stents for Femoropoliteal Artery Disease was a 479-patient multicentre, prospective, randomized study designed to evaluate the PTX stent as a treatment for PAD in the superficial femoral artery (SFA).
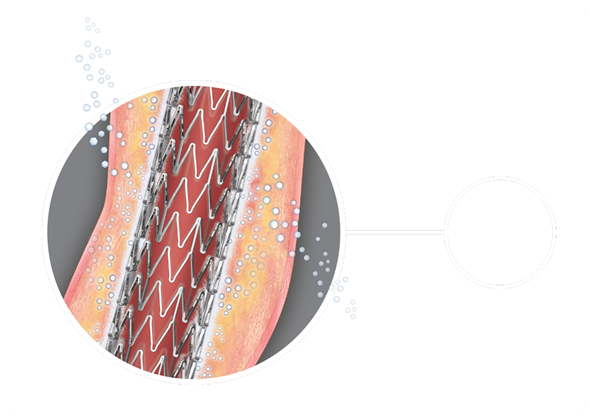
Dr. Dake presented to attendees looking for better long-term SFA results for their patients. Zilver PTX data show a 48 percent reduction in reintervention and a 41 percent reduction in restenosis compared to standard care[i]. It also showed 5-year primary patency of 66.4 percent in the superficial femoral artery (SFA) for patients treated with Cook’s paclitaxel-eluting stent. This compares to 43.4 percent patency for patients treated with standard care.
Dr. Dake chaired a symposium featuring Drs. Konstantinos Katsanos of Guys and St. Thomas’s, London; Andrew Holden of Auckland City Hospital; and Fabrizio Fanelli, Sapienza University of Rome. Live Zilver PTX cases were performed by in Leipzig and Dendermonde, Belgium.
Mark Breedlove, vice president and global business unit leader of Peripheral Intervention at Cook Medical said:
As the first and only endovascular SFA device with 5-year randomised controlled trial data, Zilver PTX is raising the standard for treating SFA disease. Physicians and patients welcome this clinical evidence proving the lasting effectiveness of drug-eluting devices in peripheral vessels. This year, we will see Zilver PTX help even more patients around the world suffering the debilitating effects of PAD.
*Dr. Dake, a global principal investigator for the Zilver PTX trial, is a paid consultant to Cook Medical regarding the research and development of medical devices.
Find out more at www.cookmedical.com
[1] Dake M. The Zilver PTX randomized trial of treating femoropopliteal artery disease: 5-year results. Presented at the Leipzig International Course (LINC); January 27-29, 2015; Leipzig, Germany.