May 19 2015
Oraya Therapeutics Inc. announced today at the Royal College of Ophthalmologists Annual Congress that U.K. ophthalmologists are the leading adopters of Oraya Therapy for the treatment of wet age-related macular degeneration (AMD), two years after the treatment was introduced in the country. Sheffield Teaching Hospitals NHS Foundation Trust alone has treated more than 150 patients in the year since the service was commissioned, with other National Health Service and private centres in England also increasing their treatment with Oraya Therapy.
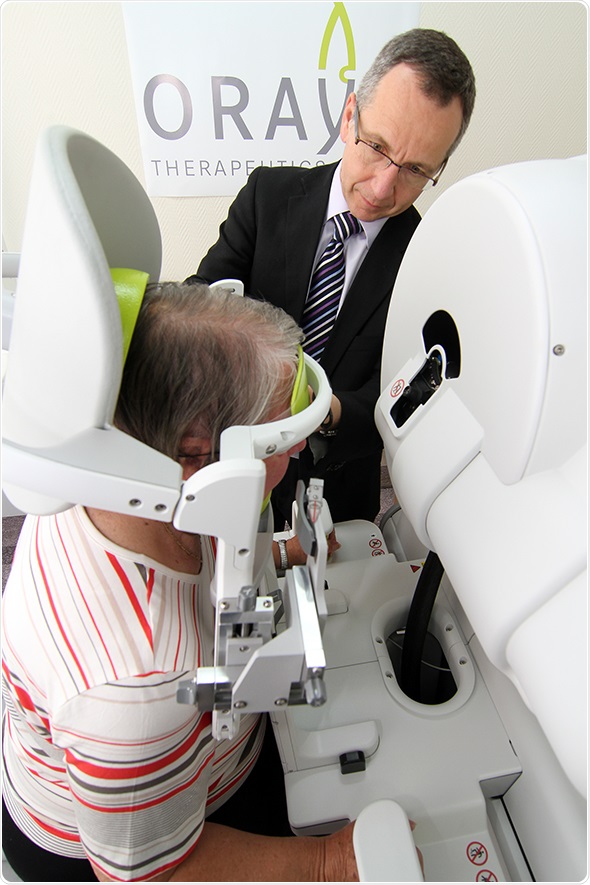
Oraya Therapy was introduced first in the U.K., followed by Switzerland and Germany. The therapy is intended as a one-time, non-invasive treatment that maintains vision with fewer anti-vascular endothelial growth factor (anti-VEGF) injections into the eye.
“We need additional therapies to anti-VEGF injections in treating wet AMD. With the availability of Oraya Therapy, we’ve been able to offer patients an adjunctive treatment that has been shown in a clinical study to reduce the number of injections required and to potentially enhance the patient's quality of life," said Mr. Christopher Brand, FRCOphth, Consultant Ophthalmologist at Royal Hallamshire Hospital, Sheffield. "In addition to benefiting patients, Oraya Therapy can reduce the burden of care in our clinics and hospitals as the number of wet AMD patients increases."
Mr. Brand, who has treated more patients with Oraya Therapy than any other physician, is scheduled to present learnings from studies of current wet AMD care within the U.K., Europe and the U.S. at the Royal College meeting tomorrow. He will present his Oraya Therapy patients’ outcomes at the EURETINA Satellite Symposia in September in a presentation, entitled “Stereotactic radiotherapy for the treatment-naïve patient with neo-vascular age related macular degeneration.”
“Over the past two years, we've had the honor of collaborating with many U.K. ophthalmologists who are working diligently and with good effect to treat wet AMD patients,” said Jim Taylor, president and CEO of Oraya Therapeutics. “We are pleased to see the integration of Oraya Therapy into standard clinical care, and look forward to establishing additional treatment centres in the coming months.”
Oraya Therapy is already available at four sites in the U.K.: Sheffield Teaching Hospitals NHS Foundation Trust, Heart of England NHS Foundation Trust, Optegra Manchester Eye Hospital and Kent Institute of Medicine and Surgery (KIMS). Outside of the U.K., Oraya Therapy is available at four treatment centers in Germany and one location in Switzerland, with an equal number in the active planning pipeline.
Oraya Therapy is a non-invasive treatment for patients with wet AMD. It delivers highly targeted, low-energy x-rays to the diseased area of the eye and is intended as a one-time outpatient procedure. The therapy has been shown in a randomized, sham-controlled, double-blind clinical study to reduce the required number of anti-VEGF injections into the eye whilst maintaining vision, and requires no post-treatment recovery period before patients can resume normal activity.
Wet AMD affects 250,000 people in the U.K. alone, and is the leading cause of blindness in people over 65 in the western world. If left untreated, it can quickly lead to loss of central vision.
They IRay® Radiotherapy system is a CE marked medical device. In the U.S., the IRay system is an investigational device and is not available for sale.