The Magstim Company Ltd, an innovative and award winning Wales-based medical device manufacturer, has received FDA 510(k) clearance to market its Magstim Rapid2 Therapy System for the treatment of drug resistant Major Depressive Disorder (MDD) in the United States. The clearance enables Magstim to significantly increase access to cutting-edge Repetitive Transcranial Magnetic Stimulation (rTMS) technology for patients and clinicians.
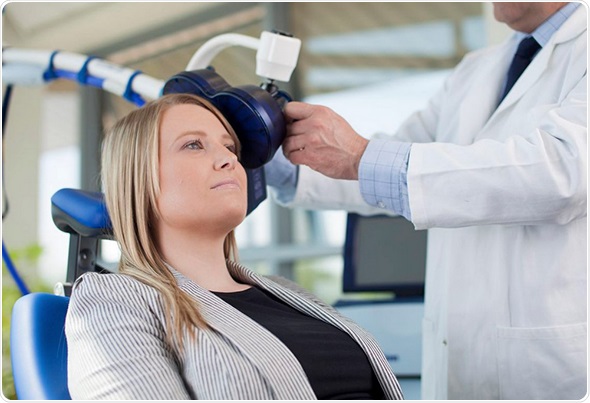
Magstim is the pioneer of TMS technology, having built a reputation for developing leading medical devices in the research field for over 25 years. The founding research team behind TMS (Sheffield University 1985) are actively involved with the company, ensuring that its technology meets and exceeds the needs of today’s researcher and clinician. The company is now committed to build on its experience by bringing its knowledge to the US clinical market.
CEO of Magstim, Mr Robin Davies commented:
It is absolutely fantastic news that The Magstim Company has been cleared by the FDA to market the Rapid2 Therapy System, which is indicated for the treatment of MDD in adult patients, who have failed to achieve satisfactory improvement from prior antidepressant medication in their current episode. This now means that we can offer quality solutions and improved choice for both the clinician and patient
Furthermore, Dr Mark George (Medical University of South Carolina, Distinguished Professor of Psychiatry, Radiology and Neurosciences, Layton McCurdy Endowed Chair), an opinion leader on rTMS for depression treatment stated: “I think that this is exceedingly good news on a couple of fronts. I think it’s great for patients because it’s always good to have competition, and more people making TMS devices should help more patients get access to the therapy. It’s also historically nice because Magstim launched the first modern uses of TMS that were pioneered in Sheffield and has been around 25 years - so it’s great that they are now present in the clinical arena. This is an excellent progression for clinicians and patients alike.”
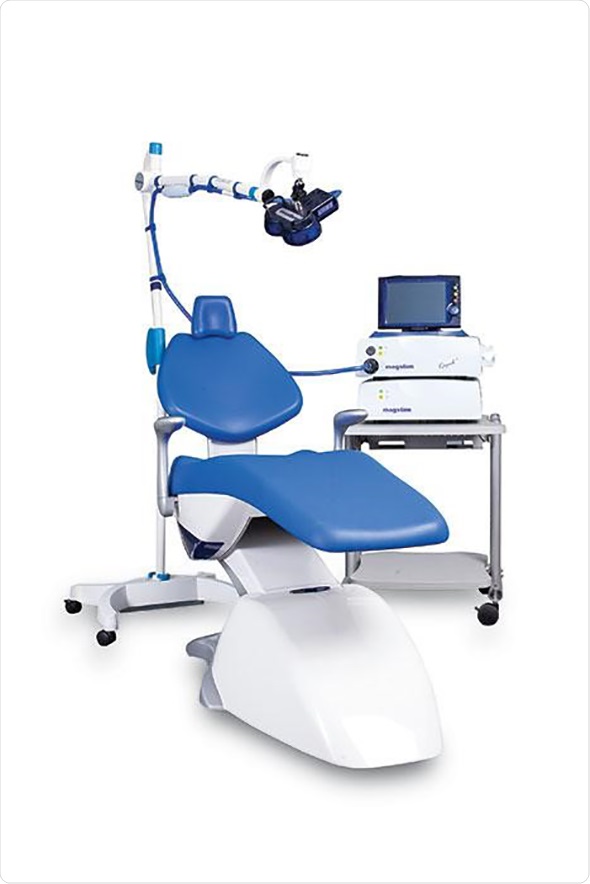
Neuro-imaging techniques have shown that people suffering from MDD commonly display reduced blood flow in the left dorsolateral pre-frontal cortex (DLPFC) and heightened activity in the limbic system, which plays an important role in regulating mood. The Magstim Rapid2 Therapy System provides an effective treatment option for patients who have failed to respond to traditional pharmaceutical medication for MDD by directly stimulating the DLPFC. Importantly, the technique is completely non-invasive and performed as an outpatient procedure, enabling patients to go about their daily lives following treatment.
Linda Carpenter, MD, Chief of Butler Hospital’s Mood Disorders Program and recognized expert in major depression and anxiety disorders at Brown University, Department of Psychiatry, Providence, Rhode Island said: "It is wonderful to learn that Magstim now joins two other companies with FDA-cleared TMS devices for treating major depression. More access to TMS therapy will translate to improved quality of life for millions who suffer with a disabling and sometimes lethal brain disorder."
Dr Alvaro Pascual Leone, Professor of Neurology, Harvard Medical School, a leader in the field of TMS, also commented: "The FDA clearance of the Magstim Rapid2 device for treatment of medication refractory depression is wonderful news for the field of noninvasive brain stimulation and particularly for patients with medication resistant depression. This approval makes more devices available for treatment of patients who stand to benefit from TMS and for whom other treatment options are limited.”
2015 American Psychiatric Association (APA) Annual Meeting
The Magstim Rapid2 Therapy System will be on show for the first time in North America on Booth No. 1405 at the 2015 American Psychiatric Association (APA) Annual Meeting in Toronto, Canada, May 16-20, 2015, at the Toronto Convention Centre. The APA Annual Meeting is the largest psychiatric meeting held, with over 12,000 anticipated attendees from around the globe, most of whom are physicians from psychiatric and other mental health disciplines.
For further information please visit https://www.magstim.com/row-en/.