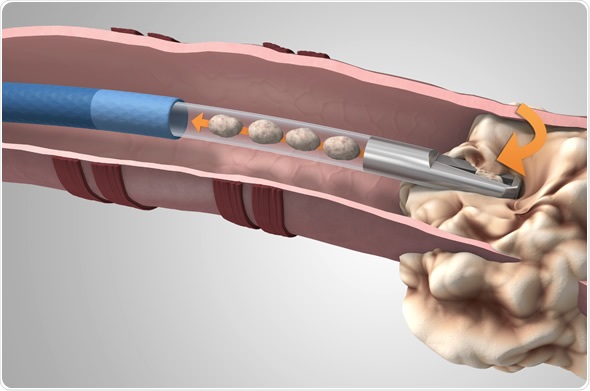
Medtronic now offers the GenCut(TM) core biopsy system, a unique lung tissue biopsy tool for use with the superDimension(TM) navigation system. The superDimension(TM) system enables a minimally invasive approach to accessing difficult-to-reach areas of the lung, which can aid in the diagnosis of lung cancer.
The GenCut core biopsy system is a unique tool that allows pulmonologists and thoracic surgeons to collect multiple core samples from lesions deep inside the lungs. With its proprietary blade design, the GenCut core biopsy system can shear and collect larger, more intact samples1, and allows physicians to provide the pathology laboratory with tissue that enables cytology, which is the microscopic appearance of cells, histology and molecular profiling for personalized medicine. With the ability to continuously sample, in one pass, physicians can potentially decrease patients' biopsy procedure times.
"Lung cancer diagnostic clinicians consistently state that tissue is the issue. When performing a biopsy, they need enough tissue for the pathologist to make a diagnosis and to ensure undamaged tissue for genetic testing and molecular analysis. As lung cancer drugs and therapies advance, the ability to provide earlier diagnosis and earlier, personalized treatment can potentially save lives," said Chuck Brynelsen, president, Early Technologies business in Medtronic'sMinimally Invasive Therapies Group.
According to the American Lung Association, lung cancer is the leading cause of cancer-related deaths in the United States2. In its early stages, lung cancer presents few, if any, symptoms. As a result, the vast majority of lung cancer patients are diagnosed in the late stages, when there is minimal chance for long-term survival.
Brynelsen concluded, "The continuum of care is the healthcare journey a patient takes from diagnosis to recovery. Our goal, across this continuum, is to diagnose earlier, intervene earlier, treat better and help patients recover faster."
The GenCut core biopsy system received U.S. Food and Drug Administration 510(k) clearance in April 2015.
About Medtronic
Medtronic plc (www.medtronic.com), headquartered in Dublin, Ireland, is the global leader in medical technology - alleviating pain, restoring health and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.