EKF Diagnostics, the global in vitro diagnostics company, announces the international launch of the Altair™ 240 clinical chemistry analyzer at the American Association for Clinical Chemistry’s (AACC) Annual Meeting and Clinical Lab Expo in Atlanta, Ga, 26-30 July. This new bench-top platform represents EKF Diagnostics’ first fully integrated chemistry system designed for the global market. Also on Booth #1018, EKF will highlight the latest additions to its range of Stanbio liquid-stable, LiquiColor® and Liqui-UV® clinical chemistry reagents.
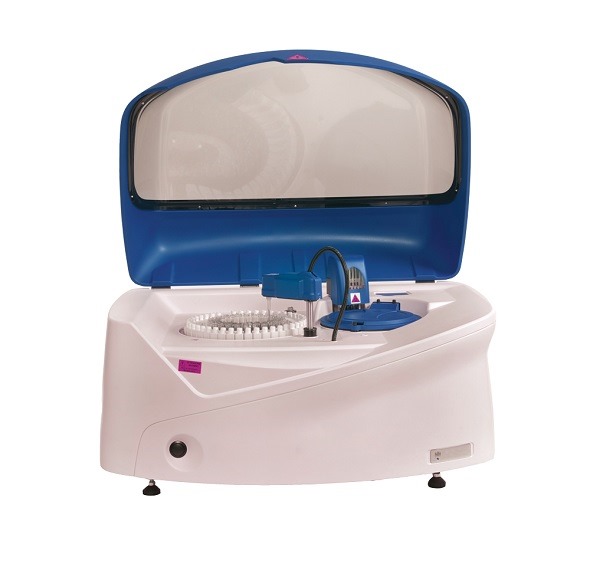
Image credit: EKF Diagnostics
The new Altair™ 240 clinical chemistry analyzer fulfills an international market need for a fully automated bench-top platform supported by the reliable and respected Stanbio Chemistry reagent menu. The fully automated system with LIS bi-directional connectivity is capable of performing up to 480 tests per hour*. In addition to being supported by EKF’s broad menu of Stanbio ready-to-use, bar coded, liquid reagents for routine and special chemistries, the Altair™ 240 also provides the flexibility to configure open channel applications for many esoteric assays.
EKF will expand the international presence of its clinical chemistry range with the Altair™ 240, as its customers have long asked for a cost-effective platform designed with Stanbio Chemistry products in mind. Built on 54 years of clinical chemistry expertise, this new platform enables EKF to offer a cost-effective solution, as either a main or back-up analyzer, to improve productivity in both the hospital and physician office laboratory environments.
“We listened to the needs of our customers and designed the Altair™ 240 to be easy to learn, operate and maintain,” said Albert Blanco, Business Unit Director for EKF’s Central Laboratory Division. “Because it runs on Windows® 7, operators can easily navigate its intuitive, touch screen menu. Features such as the capability to use bar coded, primary sample tubes, auto-rerun, auto-dilution and STAT interruption, all function to maximize the system’s overall efficiency.”
Also on Booth# 1018
EKF will also discuss the latest additions to its Stanbio clinical chemistry range, all designed for maximum stability and ease of use. This range includes a large selection of clinical chemistry assays, controls and calibrators, which are fully compatible with most major brand open channel chemistry analyzers. A notable addition is the Stanbio Chemistry Glycated Serum Protein (GSP) LiquiColor® test; a new diabetes biomarker test that provides a 2-3 week indicator of average blood glucose.
EKF will also be highlighting two of its point-of-care (POC) hemoglobin analyzers at AACC 2015 - the DiaSpect Tm and HemoPoint® H2. These deliver quick and laboratory accurate results to aid diagnoses of anemia and associated conditions within a variety of near-patient settings, including hospitals, clinics, blood banks and in challenging field-based conditions.
A hand held device delivering results in <2 seconds, DiaSpect Tm is the world’s fastest hemoglobin measurement system offering laboratory quality performance for anemia screening in all climatic conditions. The HemoPoint® H2 stores over 4000 patient results, and is designed for use in blood banks, clinics and hospitals and provides immediate lab-quality results for both hemoglobin and hematocrit - from one test. Its proprietary “soft load” cuvette holder is designed to minimize contamination of the meter.
In addition, live demonstrations of EKF’s Quo-Lab and Quo-Test HbA1c analyzers, as well as its STAT-Site® M B-HB and Lactate Scout point-of-care analyzers will take place at Booth# 1018 throughout each day of the AACC Clinical Lab Expo.
For more information on EKF Diagnostics and its range of products, please visit www.ekfdiagnostics.com.
(*Double arm functionality yields up to 480 tests per hour)