EKF Diagnostics, the global in vitro diagnostics company, announces the UK launch of the Altair™ 240 clinical chemistry analyser at the Institute of Biomedical Science Congress 2015 in Birmingham, UK, 28-30 September. This new bench-top platform represents EKF Diagnostics’ first fully integrated chemistry system designed for the global market. Also on Stand 812, EKF will highlight the latest additions to its range of Stanbio liquid-stable, LiquiColor® and Liqui-UV® clinical chemistry reagents, including an assay for Procalcitonin - an important adjunct marker in the diagnosis of sepsis.
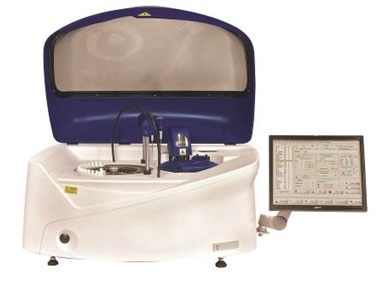
The new Altair™ 240 bench-top clinical chemistry analyser with LIS bi-directional connectivity. Image credit: EKF Diagnostics
The new Altair™ 240 clinical chemistry analyser fulfills a need for a fully automated and cost-effective, bench-top platform supported by the reliable and respected Stanbio Chemistry reagent menu. Built on 54 years of clinical chemistry expertise, the fully automated system with LIS bi-directional connectivity is easy to use, as either a main or back-up analyser, and is capable of performing up to 480 tests/hour*. In addition to being supported by EKF’s broad menu of Stanbio ready-to-use, bar coded, liquid reagents for routine and special chemistries, the Altair™ 240 also provides the flexibility to configure open channel applications for many esoteric assays.
Also on Stand 812, the Stanbio Chemistry Procalcitonin (PCT) LiquiColor® Assay is a new test enabling the quantitative determination of PCT in serum samples, EDTA or lithium heparin plasma samples by latex enhanced immunoturbidimetric methodology. PCT is a marker for bacterial infection and sepsis and has been recognized as an important adjunct marker in the diagnosis of sepsis**.
The new Stanbio Chemistry Procalcitonin (PCT) LiquiColor® Assay is fast, accurate and convenient. The test provides a precise result, which correlates well with established methodology, within 10 minutes and requires just 20 µL of sample. The reagents may be used on almost any liquid-based chemistry analyser with open-channel capability. In addition, the reagent kit, calibrator and control sets are all available separately.
Other assays from the Stanbio Chemistry range can also be seen at Stand 812 including the B-Hydroxybutyrate (B-HB) LiquiColor® Assay for ketosis testing and the Glycated Serum Protein (GSP) LiquiColor® Assay, a two-three week indicator of average blood glucose. In addition, EKF Diagnostics’ Quo-Lab™ and Quo-Test™ – devices for the accurate measurement of HbA1c – will also be showcased at IBMS, as will the Hemo Control hemoglobin analyser.
*Double arm functionality yields up to 480 tests per hour
**References:
- Müller B, et al., Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab 2001; 86(1):396-404.
- Meisner M. Procalcitonin (PCT) – A new, innovative infection parameter. Biochemical and clinical aspects. Thieme; Stuttgart, New York, 2000; ISBN 3-13-105503-0.
- Christ-Crain M, et al., Procalcitonin in bacterial infections – hype, hope or more or less? Swiss Med Wkly 2005; 135: 451-60.
For more information on EKF Diagnostics and its range of products, please visit www.ekfdiagnostics.com.
About EKF Diagnostics - www.ekfdiagnostics.com
EKF Diagnostics Holdings plc, which includes the EKF Diagnostics, EKF Molecular, Stanbio Laboratory, Separation Technology Inc., DiaSpect and Selah Genomics brands, specializes in the development, production and worldwide distribution of point-of-care blood analyzers for use in the detection and management of diabetes, anemia, lactate and kidney related diseases. Its new molecular division, EKF Molecular Diagnostics, focuses on technology used within the development of companion diagnostics, specifically within oncology. EKF products are sold in more than 100 countries around the globe, through a network of specialist distributors.
Point-of-care diagnostics: EKF Diagnostics designs and manufactures world-class diagnostic devices, as well as distributing rapid test kits for infectious diseases and pregnancy. The EKF analyzer range is used widely in GP surgeries, pharmacies, blood banks, sports clinics, hospitals and laboratories for glucose, lactate, hemoglobin, hematocrit and HbA1c measurement.
Central laboratory: EKF, through its wholly owned subsidiary, Stanbio Laboratory (Boerne, Texas, USA), manufactures a comprehensive range of clinical chemistry reagents. In addition, EKF Life Sciences (Elkhart, Indiana, USA) manufactures enzymes used in reagent development and also provides contract fermentation facilities.
Molecular Diagnostics: In March 2013 EKF set up a new division to focus on molecular and companion diagnostics following the acquisition of UK-based 360 Genomics. EKF Molecular Diagnostics’ technology, PointMan™, is capable of detecting mutant genes from tiny biopsy and blood samples and has recently entered a partnership with the world renowned cancer research center at Massachusetts General Hospital, USA.
EKF Diagnostics’ strengths lie in its multi-national research and manufacturing facilities, teams of experienced analysts and engineers in Germany, Ireland, USA and the UK, and a board led by some of the world’s foremost authorities in medical diagnosticsIn addition, live demonstrations of EKF’s Quo-Lab and Quo-Test HbA1c analyzers, as well as its STAT-Site® M B-HB and Lactate Scout point-of-care analyzers will take place at Booth# 919 throughout each day of the AACC Clinical Lab Expo.