Bladder cancer is becoming increasingly prevalent, and in 2012 was reported as the 5th most common cancer in Europe (1). While cytology screening is commonplace for diagnosing bladder cancer, a recent update to UK NICE guidelines (2) recommended the use of FISH. FISH methods have been shown to have the same specificity as cytology screening and greater sensitivity in detecting bladder cancer cells (3-4). FISH analysis for bladder cancer is also particularly useful for monitoring treatment outcome and can aid in reducing patient mortality by detecting bladder cancer recurrence up to six months earlier than other methods (5).
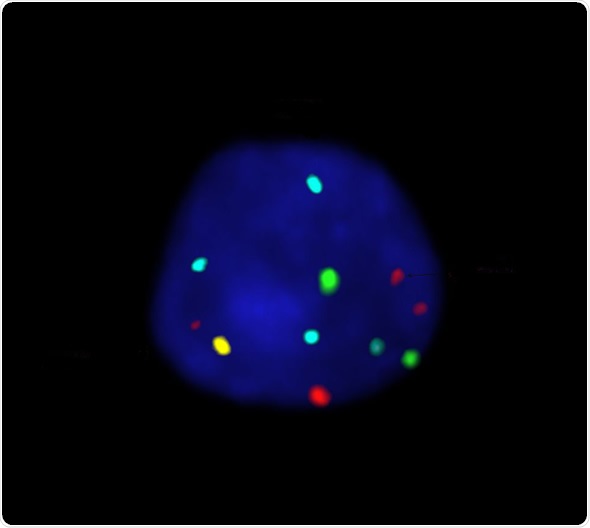 Oxford Gene Technology (OGT), The Molecular Genetics Company, has launched its CE-IVD labelled Cytocell Aquarius® P16/3c/7c/17c Probe Kit, a cost-effective, ready-to-use fluorescence in situ hybridisation (FISH) probe kit for non-invasive detection of bladder cancer — for sale in Europe.
Oxford Gene Technology (OGT), The Molecular Genetics Company, has launched its CE-IVD labelled Cytocell Aquarius® P16/3c/7c/17c Probe Kit, a cost-effective, ready-to-use fluorescence in situ hybridisation (FISH) probe kit for non-invasive detection of bladder cancer — for sale in Europe.
Carrying the CE mark for in vitro diagnostic use within Europe, OGT’s product accurately detects in urine samples the three most common aneuploidies associated with bladder cancer (chromosomes 3, 7 and 17). It also detects deletions of the 9p21.3 locus (6) containing the well-known tumour suppressor gene p16 (CDKN2A) — commonly deleted in bladder cancer (7-8). The economical Cytocell Aquarius P16/3c/7c/17c Probe Kit contains ready-to-use reagents and has an easy-to-use protocol, reducing the potential for errors and increasing convenience. Specific, clear, high-intensity signals with minimal background deliver quality, reproducible and easy-to-score results ensuring clinicians can be confident in reporting.
Dr. Lorenza Pecciarini, cytogeneticist at the Saint Raphael Hospital in Milan, who extensively tested the probe on a large series of samples, commented: “We found Cytocell’s bladder cancer probe kit easy to use, with bright results that were simple to interpret. It was straightforward to incorporate into our existing cytology workflow and was highly reproducible. This product offers an accurate and cost-effective technology for the diagnosis of bladder cancer.”
Martin Lawrie, Managing Director at Cytocell said: “The decision to expand OGT’s cancer portfolio and specifically Cytocell pathology probes, demonstrates OGT’s commitment to the fight against cancer. Supported by recent guidelines, our new bladder cancer probe kit delivers a convenient means to confident diagnoses, backed by the great customer support that OGT has become renowned for.”
For more information, please visit www.cytocell.co.uk.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Br J Cancer 2014; 110(6): 1571-8
- Bladder Cancer: diagnosis and management, NICE guideline, Published 25 February 2015
- J. Stephen Jones. Urology 2006 vol.67 Issue 3, Supplement 1 pages 35-45
- Halling et al., J Urol. 2000; Nov; 164(5): 1768-75
- Skacel M et al., Front Bioscience 2002 Jan1; e27-32
- Bladder Tumors: Molecular Aspects and Clinical Management (Cancer Drug Discovery and Development) Hardcover – 4 Dec 2010 p.202. Vinata B. Lokeshwar (Editor). David A.Levy (Author).
- Williamson MP et al., Hum Mol Genet. 1995;4(9): 1569-77
- Stadler WM et al., Urol Res. 1996;24(4):239-44
About Oxford Gene Technology
Oxford Gene Technology (OGT) provides world-class genetics research solutions to leading clinical and academic research institutions. Founded by Professor Sir Edwin Southern, and with customers in over 60 countries worldwide, OGT has a strong reputation and increasing share in the large and growing genomic medicine market. The Company’s Cytocell®, CytoSure™ and SureSeq™ range of fluorescence in situ hybridisation (FISH), microarray and next generation sequencing (NGS) products deliver high-quality genetic analysis, enabling accurate identification and confirmation of the causative variation underlying genetic disease.