In the UK there are currently over 3.5 million people who have diabetes, with a growth rate exceeding 280,000 people per year. Diabetic retinopathy is the most common complication of diabetes.
The most common cause of sight threatening retinopathy is diabetic macular oedema (DMO). This condition is characterised by the leakage of fluid from compromised blood vessels within the central retina: 240,000 people (8%) with diabetes in the UK have clinically significant DMO and 100,000 people with DMO have visual impairment.
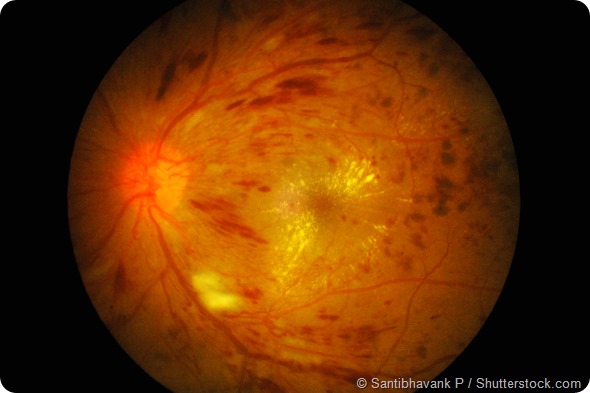
Retinal image of right eye in type I DM with finger-count visual acuity (VA) and hyperglycemia: demonstrated severe macular oedema, exudates, neovascular, and numerous flame-shaped hemorrhage.
DR and DMO develop in 90% of type 1 Diabetics and 67% of type 2 sufferers. RNIB data for 2015 quotes the number of people with Diabetes in UK as over 4 million, of whom over 1.1 million have background retinopathy and 128,725 have non-proliferative or proliferative retinopathy. With the incidence of Diabetes set to increase by 50% between 2010 and 2030, there is a tsunami of costs facing both health and social care services.
Diabetic retinopathy and diabetic maculopathy can lead to complete loss of vision. However, most sight loss due to diabetes is preventable if treatment is given early.
Can you please describe how diabetic retinopathy and macular oedema are thought to form?
There is a growing body of research which has found that diseases such as diabetic retinopathy and macular oedema are driven, in part, by lack of oxygen to the retina (retinal hypoxia). The retina uses more oxygen per unit mass than any other tissue in the body due to the fact that photoreceptors have a phenomenally high metabolic rate.
That demand for oxygen becomes even greater at night, rising by around 40% as rod photoreceptors dark-adapt. In the healthy eye, this isn’t a problem as the eye provides just enough to get by. People with diabetes commonly have microvascular damage, which can start to compromise retinal blood circulation and oxygen transport and once circulation is sufficiently compromised, the result is retinal hypoxia.
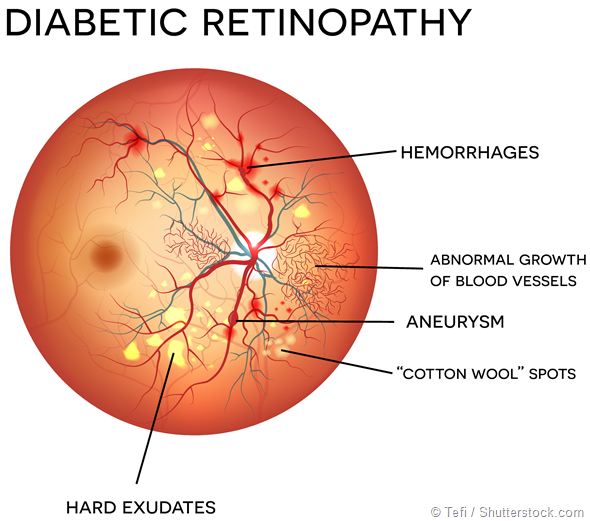
The body’s natural response to this is to promote new growth of blood vessels to compensate by releasing vascular endothelial growth factor (VEGF) but unfortunately these new vessels are weak and suffer from leakage of fluid which results in diabetic retinopathy and diabetic macular oedema.
What are the costs associated with treatment of DR?
Diabetes is a huge economic burden on healthcare systems around the world. In the UK, where we’re based, diabetes costs our National Health Service (NHS) more than £10 billion ($15.3 billion) each year – 10 percent of the total healthcare budget – and a large proportion of that cost is drugs to treat the ocular complications of diabetes.
It’s only going to get worse as the prevalence of diabetes rises, potentially by as much as 50 percent by 2030 in the UK, with obvious pharmaco- and socioeconomic implications. Anything that could reduce the number of hospital visits – and particularly, anti-VEGF injections required, could make a huge difference to patients’ lives and healthcare costs.
What are the current patient treatments for this condition?
The current treatments for DR are pan-retinal photocoagulation or intraocular injections of anti-VEGF pharmaceuticals, which are both invasive, uncomfortable for the patient and very expensive, making them treatments of last resort. They can only be given at a late stage in the development of these eye complications when patient’s eyesight is being severely threatened.
Treatment via Laser photocoagulation is a treatment that effectively cauterises the affected and damaged blood vessels in the retina. The treatment is not permanent and delays the inevitable progress of the disease and also irreversibly damages photoreceptors.
The other treatment is an intra-ocular injection of anti VEGF drugs, which works against the vascular endothelial growth factor protein produced by retinal cells. This treatment is provided within secondary care to later stage DR patients currently and costs in excess of £6,500 per eye per year.
The Noctura 400 treatment will revolutionise the treatment for Diabetic Retinopathy. Noctura 400 has been designed as a preventative care treatment to stop the progression of diabetic macular oedema (DMO) and hence retain visual acuity. The device is also a treatment for later stage at a much reduced cost to current treatments.
Clinical trial and study data has demonstrated that Noctura 400 has the potential to treat this condition at a fraction of the cost, creating the opportunity to provide an affordable and non-invasive treatment to the millions who are affected across the globe.
Can you please describe how the new sleep mask works in prevention and treatment of DR?

The PolyPhotonix (PPX) technology offers an alternative, non-invasive treatment option easily adopted (at significantly lower comparative cost base) within the current pathway. Noctura 400, with its in-built monitoring and compliance functionality, was specifically designed as a monitored home-based therapy that could form the basis of a new care pathway, bridging the gap between primary and secondary care. Future adoption of the PPX technology for the preventative care of early stage DR patients offers a route to significantly reducing numbers of patients referred into the hospital eye service.
The treatment works on the principle of preventing the rods in the eye from dark adaptation, which reduces the oxygen demand of the eye at night, preventing hypoxia and the compromised blood vessel proliferation that can result in the retina. The mask directs a low intensity light of a specific wavelength into the rod cells during sleep. It restores the rods to their daytime state, reducing the need for oxygen, avoiding the hypoxic responses and therefore preventing the progression of DR and DMO.
The Noctura 400 Sleep Mask consists of an OLED (Organic Light Emitting Diode) light housed inside a soft cushioned fabric mask, designed to be worn at night, to deliver a precise dose of light therapy during a patient’s normal hours of sleep. The mask is programmed to administer the correct dose of light each night as part of a continuing therapy; the mask also measures patient compliance.
At the end of the allocated period (usually 12 weeks), the mask is returned for analysis and a replacement mask is provided. The collected compliance data allows the clinician to compare how regularly the mask has been worn, with changes in vision and the condition of the disease.
What advantages are there to using the sleep mask over the current treatments?
Today, treatment is only available when the DMO becomes clinically significant or shows progression to the fovea. Laser photocoagulation treatment is the standard of care when the DMO becomes clinically significant. Although laser treatment reduces the risk of moderate visual loss by 50% at this stage, it is not effective in restoring best corrected visual acuity (BCVA) and has significant side effects that impact on the patient’s quality of life.
Another treatment option is inhibitors of vascular endothelial growth factor (anti-VEGF) which are injected into the eye. These treatments are costly and cause significant burden to patients, their care-givers and the healthcare system.
The Noctura 400 device offers a new intervention opportunity within the current NHS care pathway and also as a new intervention supplied and monitored within a new primary care pathway, both having the potential to deliver significant patient and NHS resource/cost benefits.
Will this new form of treatment be usable for all DR patients? Is it as effective as Laser Photocoagulation or Intraocular Injections?
The Noctura 400 Sleep Mask is a Class 2a medical device, which has CE certification, and its design and manufacture meet the standards of ISO13485. CE certification requires proof of efficacy as well as safety and as such is supported by a comprehensive technical file.
The device has completed a number of trials and there are number still in progress. Clinical trial have shown that it is highly effective when compared to current treatments showing a significant reduction or clearance of cysts in the subfield of maximum pathology. PolyPhotonix intends to publish clinical results early next year.
SLEEP MASK COULD SAVE SIGHT.
What clinical research is PolyPhotonix conducting into this treatment?
Noctura 400 has successfully completed clinical safety and efficacy trials and is currently in trial in over 35 NHS hospital clinics with more than 160,000 hours of recorded use.
PolyPhotonix has had excellent support for the development of the Noctura 400, with peer reviewed grants totaling £14 Million over the past 5 years from Innovate UK, NHS, SBRI Healthcare and other sources. Much of the early safety & efficacy research and trials was undertaken at Liverpool University through Innovate UK*, KTP and KTN funding. Phase III (CLEOPATRA) trials are underway in a number of other NHS hospitals (15+) as is the CANDLE (NICE MTEP) in another 15 NHS hospitals. Early product design work was undertaken by Northumbria University.
*Innovate UK is a government-backed SME (small & medium-sized enterprise) incubator. They are actively preparing for Innovate 2015, the annual innovation conference showcasing other promising healthcare companies such as PolyPhotonix.
What are the future research goals that PolyPhotonix are striving towards?
PolyPhotonix, a rapidly growing SME, is committed to developing NE England based manufacturing and has established a significant part of the supply chain in the NE. PPX research, develop and manufacture photonic devices for various applications, including healthcare and we have a number of research programs underway in the UK, unfortunately we cannot discuss them publicly.
The mask is also being adopted by a number of forging health care services.
PPX is also developing high volume manufacturing processes for the manufacture of OLED devices.
The Noctura 400 Sleep Mask is currently available to patients privately through approved optometrists as well as through our national provider, The Outside Clinic, who provide a home optician service. PolyPhotonix also donate 5% of UK revenue to Fight for Sight, the UK eye research charity.
Where can readers find more information?
On the Noctura web site there are a number of patient testimonials included as well as an OCT scan from a patient who has used Noctura 400, showing the before and after scans (during a 6 month period).
There are some good links and information on www.noctura400.com and www.polyphotonix.com
About Richard Kirk_thumb.jpg)
With over 15 years’ experience in the field of medical research and printed electronics, Richard is well recognized as a pioneer in material science and its applications.
He is credited for many world’s first’ applications using inorganic and organic light emitting materials. He is a regular keynote speaker internationally and sits on a number of industrial and government advisory boards. Richard and his company have won many international and national awards for innovation, research and business.
With an early life as a successful artist in based in France, Richard has a thorough understanding of the creative process and a unique view on the development of markets for innovative research.