Novartis announced today new results for Cosentyx® (secukinumab) showing no further progression in joint damage in 84% of patients with psoriatic arthritis (PsA). In addition, Cosentyx maintained a treatment response in joint and skin disease, physical function and quality of life in patients over two years of treatment.
These results from the extension phase of the FUTURE 1 study were presented at the 2015 Annual Meeting of the American College of Rheumatology (ACR) in San Francisco, United States.
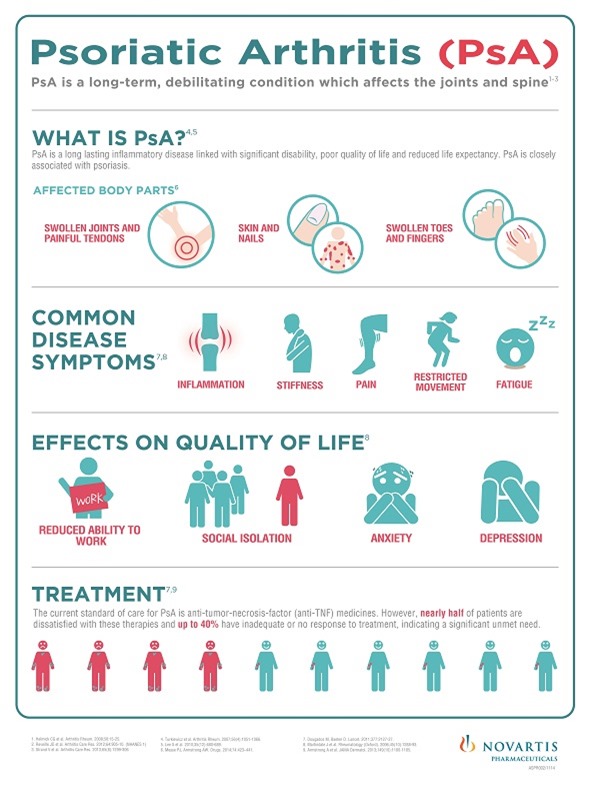
Cosentyx is the first of a new class of medicines called interleukin-17A (IL-17A) inhibitors to demonstrate efficacy in Phase III studies in PsA – a life-long inflammatory disease that affects the skin and joints. If not treated effectively, it can lead to irreversible joint damage and disability caused by years of inflammation.
“Psoriatic arthritis patients need therapies that can prevent the progression of this debilitating disease. In this two-year study, Cosentyx showed no further progression in joint damage in over 80% of PsA patients while maintaining improvements in joint and skin disease, physical function, and quality of life.”
“These results show the potential for Cosentyx to create an important new option for the treatment of psoriatic arthritis.”
Vasant Narasimhan, Global Head of Development, Novartis Pharmaceuticals.
New medicines with an alternative way of working are needed as many patients do not achieve an adequate response from current treatments, such as disease-modifying anti-rheumatic drugs, non-steroidal anti-inflammatories or anti-tumor necrosis factor (anti-TNF) therapies. Many patients do not respond to or tolerate these therapies, with approximately 45% of PsA patients dissatisfied with their treatments.
These results from the FUTURE 1 study represent the longest Cosentyx Phase III study in PsA to date. Responses in joint and skin disease, physical function, and quality of life at Week 24, were maintained over two years.
After two years of treatment, 67% of patients (n=202) treated with Cosentyx 150 mg achieved the standard treatment goal of an ACR 20 response (American College of Rheumatology response criteria).
In addition, 84% of patients showed no further progression in joint damage as shown by x-ray assessment. Cosentyx was well tolerated with a safety profile consistent with that observed in previous studies.