Novartis announced today late-breaking two year results for Cosentyx® (secukinumab) showing up to 80% of patients with ankylosing spondylitis (AS) had no radiographic progression in the spine on x-ray assessment. This is the first time that data on structural spinal progression in AS have been presented for an interleukin-17A (IL-17A) inhibitor.
In addition, Cosentyx showed a sustained response in improvements of signs and symptoms, physical function and quality of life in AS patients over two years. These results from an extension phase of the MEASURE 1 pivotal study were revealed at the 2015 Annual Meeting of the American College of Rheumatology (ACR) in San Francisco, United States.
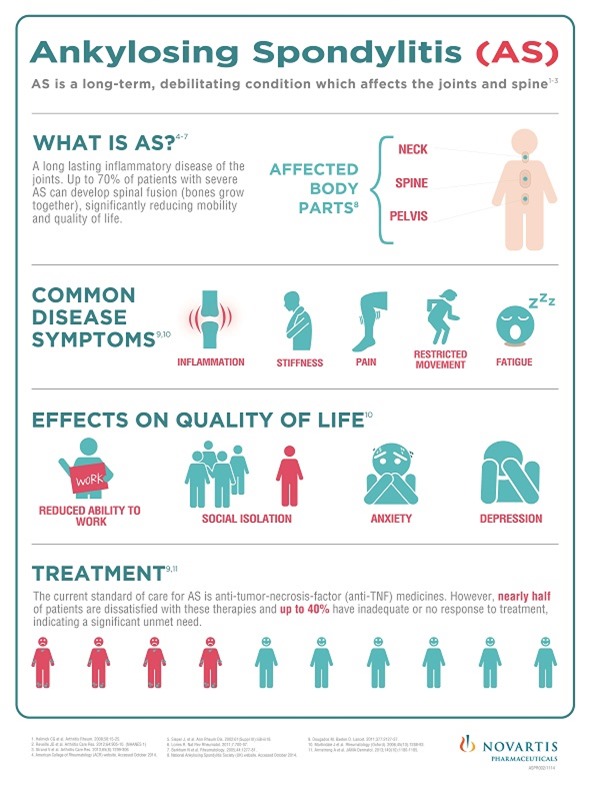
Cosentyx is the first IL-17A inhibitor and the first biologic treatment other than the current standard of care — anti-tumor necrosis factor medicines (anti-TNFs) — to demonstrate efficacy in Phase III AS studies. AS is a life-long and painful inflammatory disease that can cause irreversible joint and/or spinal damage for patients if not treated effectively.
“Many patients with ankylosing spondylitis will go on to experience stiffening of the spine. Cosentyx has shown the potential to reduce structural damage of the spine in ankylosing spondylitis patients across two years,” said Vasant Narasimhan, Global Head of Development, Novartis Pharmaceuticals. “Cosentyx is a real contender for becoming a new standard for treating ankylosing spondylitis patients.”
These results are important because up to 70% of patients with severe AS develop spinal fusion (where the bones grow together) over 10 to 15 years, which significantly reduces mobility. New treatment options are also urgently needed as many patients do not respond adequately to current medications such as non-steroidal anti-inflammatories and anti-TNF therapies with up to 40% of patients not responding sufficiently to the latter.
Data from 125 patients presented for Cosentyx 150 mg showed that 74% achieved an ASAS 20 response (Assessment of Spondyloarthritis International Society response criteria) at two years. The study enrolled patients who had either never taken, or who had previously been treated with, the current standard of care biologic, anti-TNF therapy. Quality of life and physical function scores were also maintained over two years. Cosentyx was well tolerated over the two year treatment period with a safety profile consistent with that observed in previous studies across multiple indications.