There are two types of protection, sample protection keeps the sample away from contaminants in the air; and operator protection, which protects the scientist from harm from whatever they’re working on. With a biological hazard, if you’re just doing sample protection the worst that can happen is the sample gets contaminated and your work is ruined.
 K_E_N | Shutterstock
K_E_N | Shutterstock
However, with operator protection, safety is very important because the worker is going to have a problem. In a microbiological or a class two hazard containment environment, maybe a university research department, or oncology, the researchers will work with infected samples.
The samples could have TB, hepatitis or various other infectious contaminants. We have some customers that work with Anthrax, others who work with the flu virus. Novartis in particular are manufacturing flu and have a seed lab, with their department in Liverpool. We’ve got our equipment in there, if the equipment failed, the operators would most likely become infected.
Labs like Novartis’ lab take the flu virus like H1N1 and many other different strains that you get every year, which normally come from China. They take a live virus and they need to grow it and make more of it before they can go on and do some manufacturing.
If that cabinet fails these people are going to get flu, TB, hepatitis, whatever they’re working with, because it will blow it around the lab.
It's really quite serious stuff when you know it's very important on the other side. There are also the chemical hazards which are equal or sometimes even more hazardous. Their function is about the same, they'll be working with toxic chemicals such as formaldehyde which has got a very low thresh hold for safety, half a part per million is the limit.
The whole industry surrounding our products is driven by health and safety. The laminar flows, the microbiological safety cabinets, the fume cupboards, they are all covered by British and/or European standards, the SEM Standards.

That's already in place and you have to adhere to those rules with have stringent tests. That restricts the level of design you can have with them because, it’s got to meet the standards of the law. The designs become quite limited based on what can actually be done, it’s difficult to be radical with the design.
Can you please give me a brief overview of the type of products that Cantel Medical produce?
They're all to do with making your air clean in some way. Whether it’s from removing contaminants that are dirty and making sterile air with our HEPA filter or whether you are moving chemicals and you're making air clean by removing the chemicals from the air with carbon filters, maybe also by exhausting it to the roof. We are either doing operator protection or sample detection or both.
We also offer service and maintenance on everything we sell, whether it be our brand or another brand. We have a team of 12 engineers throughout the UK, based regionally.
From left to right: fume cupboard testing, airflow testing, DOP testing.
Also, recently decontaminating the cabinets and the filters was allowed with formaldehyde gas, but that has recently become illegal. We now offer an alternative which is vaporized Hydrogen Peroxide gas. That takes expensive equipment, so we now offer that as a service to all of our customers, we have a solution immediately.
What research and commercial sectors are currently using this equipment? What type of research is typically conducted using these products?
Most of the products are quite generic and can be used in almost any setting. Academic universities use them, that's probably our biggest market sector. NHS Hospitals, specifically, pathology and microbiology departments. A lot of pharmaceutical companies use them in their laboratories. Most people who have a laboratory will probably have a requirement for one of our units.
Laminar flows, give sterile air sample protection that would be used more for microbiology or even electronics companies, semiconductor manufacturer. We have some products in Hewlett-Packard and other similar companies that make sterile aspects of circuit boards, semiconductors and microchips.
Animal research is a quite big market with regards to operator safety from the dust and dander and allergens that come off animals. Also some research requires that the animals are kept sterile. Sometimes it's a dual function, they want just operator protection, sometimes they want just sample protection or maybe they want a mix of both.
For example one of the cabinets we produce is a class two microbiological safety cabinet, the samples, the tissue cultures and cell cultures need to be sterile so they don't get infected. They’re also probably carrying a virus at the same time, which you don’t want to infect other samples. It would be a dual role of operator and sample protection in that case.
What features do your products offer that ensures the safety of researchers and medical health professionals?
All of our cabinets adhere to regulatory guidelines, we tend to exceed them in a lot of cases because we always made them to the old British standard, which is more stringent. For example, with the class two microbiological cabinet, the British standard requires a double HEPA exhaust filter, while the European standard only requires one.
Most of the products we make are either ducted up to the roof via a chimney stack with a fan, or they are recirculating. They have to operate to the same level of operator protection in either case. In the case of the ducted system, if something does go wrong at least it’s being pumped out onto the roof. The ones that are recirculating, these are more important because if they fail they are exhausting into the room that you're working in.
If you are working in the same room with the air and something fails it's just going to blow it around the room and you may as well just work on an open bench. It would make no difference. In fact it’s probably worse in a cabinet because the air flow will accelerate the movement of air rather than leaving it stable on a bench.
The British standard requires a double HEPA exhaust filter for the recirculating type two cabinets. That gives a double chance that any failure should be half, because there are two barriers rather than one. Yet the European standard is the BSEN12469, which supersedes the old BS5726 and 5295, which requires only one requires on HEPA exhaust filter. You find that most go for the double, for a safer approach.
What is the importance of the HEPA filter? What other filters are there?
The two types of filter we use on our recirculating cabinet are a real mix and match depending on the application. For example, a HEPA filter is purely for particulates, airborne particulates. That could be dust particles, virus particles, bacterial spores, whatever it may be that you don't want blowing around, it's an absolute filter. They are rated to .3 microns. 99.997% efficiency.
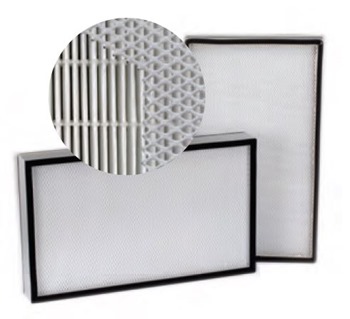
We make a slightly higher one, an ULPA filter which is an ultra-low particular air filter. That would be more for the people like Hewlett-Packard with the semiconductors that they’re working on, because they want absolutely clean air. It is a 0.12 micron filter and 99.9998% efficiency. It's just the next level up.
We offer both as an option in all of our cabinets depending on the application. A HEPA filter is used for particulates whether it's making for the air sterile or it is removing things from the air in the first place on the way out. It could be incoming or out going. It just depends on the type of cabinet.

We also have carbon filters, which are activated charcoal, more used for chemical containment. In our research with our fume cupboards, our standard activated carbon works with all of your organic compounds because the organic matter will readily bind to the carbon.
However, the inorganic acids such as the ammonia based compounds and sulfur based compounds, won't bind so we have to impregnate the carbon with another chemical to get a reaction between those two chemicals to extract the fumes.
We have some customers that may have a fume cupboard in, for example, MedImmune in Liverpool. They want it to work with a big ducted fume cover, their fumes are not really an issue but they’re working with types of cytotoxic powders, which are not allowed to be exhausted into atmosphere, onto the roof because it would just blowing into the local environment.
They won't be degraded or diluted enough and you'll end up with a pile of white powder on the roof, which will be toxic. So we created a special unit, a normal ducted fume cupboard for the fumes, but with a double HEPA phase change system for the cytotoxic powder.
If the client is working with a biological organism, such as a virus or a bacterial spore, you can kill it with various gases and sterilize it before you service the filter or remove the filter for disposal etc.
If you're collecting toxic powders, there's nothing you can do to stop them being toxic. They're just trapped in a filter, the same as a vacuum cleaner. The same idea, the same filter that you have in a Dyson.
What we do in those cases, we have a "Safe Change System". There are two HEPA filters, the second one is a slave. The primary one does all the work, which means you can change the primary filter with the cabinet running, with containment. Any contaminants are still contained by the secondary slave filter, which stays in there forever and doesn't get changed.
It's the same as the animal applications, where you've got fur and dust. They're allergenic. You can't stop the particles being allergenic, it's still there. If you drop the filter on the floor and bash it, all of the dust that you've been collecting for the past two years will just fly off. Which is obviously very bad for the operator or the engineer that's working on it. We use a Safe Change System for these applications as well.
Focusing on the downflow tables, can you give me a brief overview of how Cantel Medical’s tables differ from competitors’ products?
Yes. It's a different market to the products that we've already discussed like cabinets and so on, which are quite generic and with many different competitors. Down flow benches are a very niche market, specifically: animal dissection, mortuaries, histo-pathology and pathology in hospitals, basically for organ dissection.

There are probably only three or four manufacturers in the UK, maximum and around the world, no more than twenty that do this sort of thing.
Where we differ from our competitors, companies such as AFOS and LEEC. They tend to offer standard products, maybe a modular sort of system, take a one meter piece and add it to another half meter piece a bit like Lego.
We offer a quality bespoke service to our customers, they can design it from a blank piece of paper and have it exactly as they wish, we're the only company that offers that solution. It's not at a premium cost, we're still around about the same price as the people who offer the modular system, but the customer can have what they want.
Customers want the design like a production line, they can have it laid out in the way in the way that they want it. So that it fits into their establishment. Rather than trying to make their work fit round the unit, that's how we differ.
It’s a growing market, within the NHS alone, the numbers of samples and of patients is rising all the time. They may be processing a hundred thousand samples a year, maybe more.
Currently downflow tables are not regulated in the same way that fume cupboards are, can you give me a brief overview of the changes that are likely to take place to these regulations and how this will affect the industry, including Cantel Medical?
There are currently no standards whatsoever.
I think as we are now speaking to the health and safety executive (HSC), helping them to write some guide lines. I think we should be okay, with many of our products, and because we are a British manufacturer, we tend to over-engineer, there is a lot of redundancy.
We always have a lot of excess air flow available, if we need it we can increase things. We set our products to have the highest airflow in the industry. Our competitors have a minimum air flow as a down flow into the table at 0.3 meters per second. We set ours with a minimum of 0.4 or 0.45.
We have more airflow in the first place, which gives better containment, and we can go up from there as well. The new regulations are probably going to help us because some tables are going to fail miserably. We know of tables in the market that are struggling to do 0.3 and customers complain that they can smell chemicals which, they will be able to. We’re quite keen to work with the HSC to achieve some standards.
Because there have been no standards for any of it, the products were developed by people in our industry as a solution to our customer's requirements. The technology with the filtration is based on the fume cupboards, which are a proven technology. It's all adapted across but there's nothing specifically saying "If you put this filter into a down flow table it must do this". It's just been adapted across by the manufacturers who know how to make it work.
What other specialized products do you offer in the downflow table range?
We're one of the few manufacturers to do recirculating down flow tables. Most of our competitors do ducted. It’s a large box with an extraction system to the roof, it's quite simple.
We're also one of only a couple manufacturers in the world that use activated charcoal and/or HEPA filters in our downflow tables, like we do in our recirculating fume cupboards. We offer that, together with this whole custom design for the down flow tables.
A lot of customers cannot or are not allowed to duct up to the roof. Maybe they are in the basement of an eleven story tower block and the costs are prohibited to get the ducting all the way up there.
There are planning regulations, a lot of local planners especially in London, won't be allowed to put three or four big, horrible, grey chimney stacks at the side of the building, in the first place. Retrofitting it internally is not an option, unless it's a brand new building. We offer a solution, just park it wherever you desire, connect it to the plumbing and waste and it will work, it's safe.
What does the future hold for Cantel Medical?
The future's looking really good. We've got some new products coming on line, we've got a brand new class two cabinet coming out in March next year, with a new standard that's been written with GAMBICA.
We're sat on the board, together with some other manufacturers to write a new standard for recirculating fume cupboards. That'll also drive a new cabinet off the back of that to comply to the new standard that we helped write. That will be coming out within the next eighteen months.
The business is growing rapidly with the down flow tables, as well. There are lots of customers that have products older than ten to fifteen years old that need replacing. The HSC are probably going to take eighteen months to get the standard out because it needs lots of approval and sign off by various parties, as well as time for consideration.
Once that happens we'll see a massive boost in the market for down flow tables because I think most people will find they’re not compliant.
Where can readers find more information?
Cantel Medical
About Gary Broomhead
 I’m currently the Head of Sales at Cantel Medical. Originally I came from a scientific background, I first started work with Astra Zeneca in 1992 as a research scientist. In 1995 I moved to work for Ethicon, who make medical sutures and other surgical equipment. I worked in their quality control division, doing product assurance testing.
I’m currently the Head of Sales at Cantel Medical. Originally I came from a scientific background, I first started work with Astra Zeneca in 1992 as a research scientist. In 1995 I moved to work for Ethicon, who make medical sutures and other surgical equipment. I worked in their quality control division, doing product assurance testing.
In 1996 I began work in the University of Oxford, anesthetic department in the John Radcliffe Infirmary. There I was a research scientist studying myocardial ischemia in relation to anesthetics and analgesics for use in surgery.
I then moved to the University of Manchester, department of oncology as a researcher during 1997. During 1999 I moved to work with Wolf Laboratories for a year in London, who have now become a dealer for Cantel Medical. I then went to work for Novartis in vaccine research, development and testing.
At the end of 2001, I moved into sales with Labcaire for three years, before working with Wolf Laboratories again and Binda the German incubator and climactic test chamber company for four years. I then moved to work with Lab Mode for four years before returning back to LabCaire, which has been bought by Cantel Medical.