While there are a range of reports that different foods and food groups can increase or decrease your risk of cancer, these associations are very difficult to scientifically verify.
However, there is research that has demonstrated a strong connection between environment, nutrition and cancer. Studies have shown that African and Asian women have a significantly lower incidence of breast cancer than those in western nations.
However, for those African and Asian women that emigrate to a typical western nation and consume the local diet, their incidence of breast cancer increases to that of the Caucasian population.
Why do you think a change in local diet would increase the risk of cancer?
Recent investigation by the Centenary Institute has focused on narrowing down the key nutrients required by cancer cells to expand and multiply, and our research highlights how this link can be therapeutically exploited to efficiently “starve” the cells.
The Western diet includes many eating habits that have been reported to increase the risk in breast cancer. One example of this is the higher consumption of meat and dairy products, which have high levels of amino acids such as glutamine.
Many cancer cells, unlike normal cells, divide and grow relying on glutamine instead of glucose. We have found that some breast cancer cells absolutely require glutamine for their survival, and that by blocking ASCT2 (a glutamine pump) we can stop their growth and starve them of the critical nutrient glutamine.
Tumor cells under microscope labeled with fluorescent molecules
However, glutamine is naturally produced in the body, so a change in diet to exclude or reduce glutamine alone, is unlikely to be sufficient to starve cancerous cells. The Centenary Institute has been investigating drugs that inhibit these nutrient pumps allowing glutamine to be taken up, inhibiting the growth of cancer cells.
How does the amino acid pump (ASCT2) work? Why would blocking the pump starve the cancerous cells?
ASCT2 is a cell surface transporter that pumps amino acids across the cell membrane. Amino acids are the building blocks of proteins. One of ASCT2’s primary substrates is the amino acid glutamine.
Although a non-essential amino acid in normal cells, the demand for glutamine is dramatically increased in fast-growing cancer cells to support their rapid growth, and many cancer cells become conditionally dependent on it for their growth.
Investigating how breast cancer cells hijack nutrient transporters to fuel their increased glutamine demand, we found more ASCT2 glutamine pumps on the surface of breast cancer cells than normal cells. This increased number of AST2 pumps means the cells were able to bring more nutrients into the cell, fuelling their growth.
Furthermore, blocking the main pump, ASCT2, stops the tumors from growing, which suggests the ability to “starve” cancer cells could lie with ASCT2 (SCL1A5) nutrient pump.
Why could this research be so important? How does this affect those diagnosed with triple negative breast cancer? Can you give me a brief overview of why triple negative breast cancer is so difficult to treat?
The breast cancer cells that respond most strongly to blocking ASCT2 belong to a highly aggressive subtype known as “triple-negative” breast cancer, and these patients have very poor prognosis. This research offers new hope for those breast cancer patients.
Triple-negative tumors, making up around 15% of all breast cancers, lack expression of the three cell-surface receptors that drive the majority of breast cancers: estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2).
Patients with tumors expressing these receptors can be treated with targeted drugs such as tamoxifen and Herceptin, however, since triple-negative breast cancers lack these receptors, patients are treated predominantly by surgery, chemotherapy and radiotherapy.
There is a growing need for targeted therapies in this challenging subset of breast cancer, as these patients have a lower 5-year survival and increased cancer recurrence compared to patients with targetable receptors.
What makes a compound which blocks ASCT2 a good candidate for a targeted therapy? What would make the compound target cancerous and not healthy cells?
Targeted therapies strive to be more specific at killing the tumor cells, with less toxic side-effects on normal cells. This current research suggests ASCT2 is a very strong candidate for a targeted therapy, as it is expressed highly in the triple-negative breast cancer cells, but is not required for the majority of normal cells.
Importantly, mice lacking ASCT2 seem to develop normally, suggesting ASCT2 is not required for normal cells to function.
Our research also found that less aggressive breast cancers were unaffected by ASCT2 blockage, whereas these fast-growing, highly aggressive triple-negative breast cancer cells were very sensitive to glutamine starvation.
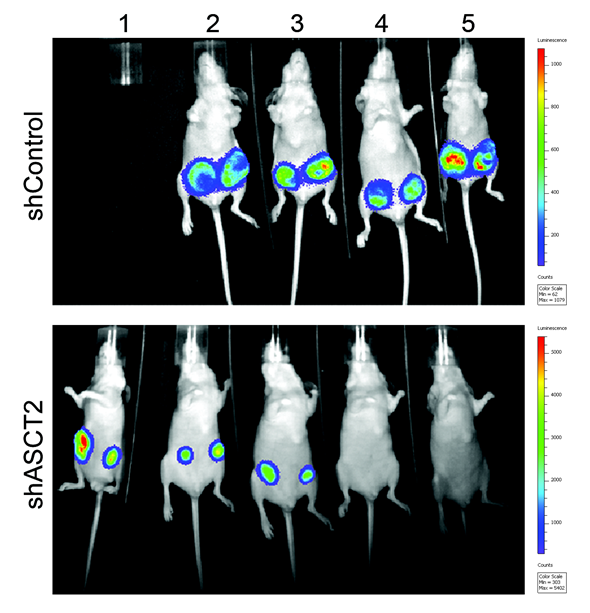
Blocking ASCT2 was able to prevent the growth and survival of these triple-negative breast cancer cells both in the laboratory and animals models of triple-negative breast cancer.
What other applications are there to this research outside of breast cancer? What does the future hold for your research?
Our research has demonstrated that “starving” cells by blocking ASCT2 is effective in preventing the growth of melanoma, prostate cancer and breast cancer cells. Other labs around the world have shown ASCT2 is important in a range of other cancers.
We are developing new compounds that can target ASCT2, and we are working to develop these compounds into drugs that are suitable for use in patients. If this development pipeline is successful, these new ASCT2-targeted drugs could be tested in clinical trials in as little as 3 years.
This study was conducted using cell and animal models alongside primary analysis of triple-negative breast cancer patient samples, and was recently published in the world-renowned cancer journal, Oncogene.
Further Reading
To find out more about the Centenary Institute’s Origins of Cancer Program, visit www.centenary.org.au
Oncogene
About Prof Jeff Holst
Associate Professor Jeff Holst joined the Centenary Institute in 2006, after completing a 3-year postdoc at St Jude Children’s Research Hospital in the USA, where he was studying T cell development and function.
He formed the Origins of Cancer Laboratory in 2008, setting out to try and understand how cancer cells obtain nutrients, and whether this could be targeted therapeutically.
He joined the faculty at Centenary in 2015, and is currently funding by Movember/Prostate Cancer Foundation of Australia, National Breast Cancer Foundation, Cancer Council NSW and The University of Sydney.
Jeff’s team have has discovered how leucine pumps are regulated and control cancer growth in prostate cancer (Cancer Research 2011, Journal of the National Cancer Institute 2013), as well as the role of glutamine transport in prostate cancer (Journal of Pathology 2015) and melanoma (International Journal of Cancer 2014).
Current projects are focusing on therapeutic targeting of these nutrient pumps in triple-negative breast cancer, prostate cancer and melanoma.