microRNAs (miRs) are small endogenous noncoding RNA molecules (20–23 nucleotides) derived from imperfectly paired hairpin RNA structures naturally encoded in the genome that act specifically as triggering molecules to control translational repression or mRNA degradation.
They regulate 10–30% of all protein coding genes, targeting amino acid coding sequences, as well as promoters of gene expression and long-non-coding RNAs.
This remarkable machinery involved in gene regulation processes is evolutionarily conserved and involved in many biological processes such as cell proliferation, differentiation, apoptosis, metabolism, development, aging and cancer.
What methods are currently used to deliver microRNAs for cancer treatment? Why is this a difficult task?
microRNAs show high potential for cancer treatment, however one of the most significant bottlenecks in enabling miRNA effect is the need for an efficient vehicle capable of selective targeting to tumor cells without disrupting normal cells.
Even more challenging is the ability to detect and silence multiple targets simultaneously with high sensitivity while precluding resistance to the therapeutic agents.
What are the main disadvantages and limitations of current techniques?
Poor miR in vivo stability, nonspecific biodistribution, modification of endogenous RNA machinery, and undesired side effects are the main limitations. However they may be overcome by an efficient and sustained delivery platform.
The delivery of miRs has been realized using inorganic nanoparticles (for example, gold, magnetic), liposomes and lipids, micelles, peptide nucleic acids, packaging RNA and polymeric nanoparticles. However, miR dissociation from the vehicle, low vehicle stability and inefficient targeting, all lead to poor silencing efficiency.
Please can you outline your recent research into a new way to deliver microRNAs for cancer treatment?
To address the limitations associated with current miR delivery vehicles, we developed an RNA-triple-helix structure for modulating the expression of endogenous miRs in cancer in vivo.
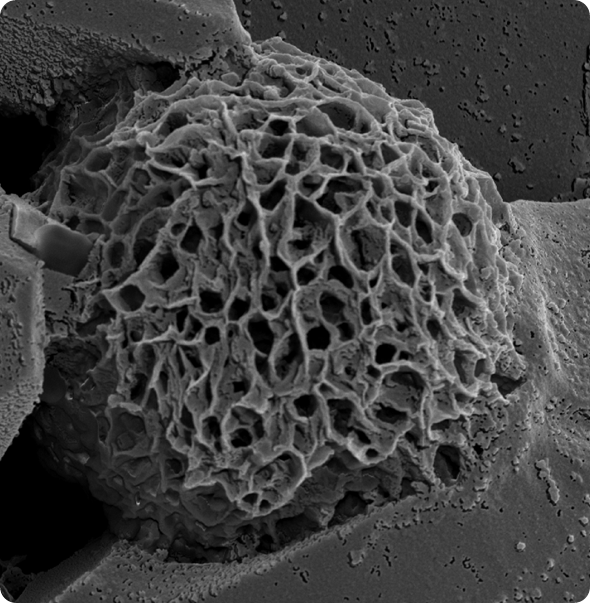
RNA triplex nanoparticle. © Dr Joao Conde
Two miR oligonucleotides forming a triple helix were used for an in vivo miR inhibition strategy (antagomiR, a small synthetic ssRNA used to inhibit an oncomiR) and a miR replacement therapy (miR mimic, a mature miR duplex composed of both sense and antisense strands used as tumor suppressor) in an orthotopic triple-negative breast cancer (TNBC) mouse model.
TNBC is characterized by a lack of progesterone, estrogen and HER2 receptors. Thus, TNBC is not responsive to conventional hormonal therapy (such as tamoxifen or aromatase inhibitors) or therapies that target HER2 receptors, such as Herceptin (trastuzumab), which is a biological macromolecule for targeted therapy. Hence, TNBC can benefit from gene therapy approaches, including endogenous miR modulation.
We further devised that device efficacy would be enhanced if stabilized in a helical structure and protected by an adhesive hydrogel formed in situ that coats the tumor. Hence, this study reports on the development of a novel RNA-triple-helix hydrogel scaffold through programmable self-assembly of the two miR sequences to provide a stable and efficient nanovehicle for in vivo miR local delivery.
How did the delivered microRNA compare to standard chemotherapy treatments?
Bioimaging of mice and tumor size measurements revealed that only the RNA-triple- helix hydrogel scaffolds were able to promote efficient and sustained inhibition of tumor progression, with almost 90% tumor size reduction 13 days after hydrogel disk implantation.
Conversely, the chemotherapeutic drugs such as DOX- and PTX-loaded hydrogels showed only a 25% and 35% reduction in tumor size, respectively.
Were you surprised by these findings?
Yes, although we worked for several years with this local platform and knew that had huge potential in terms of efficacy.
Do you think this approach could be used for delivering other types of RNA and DNA?
This approach can be implemented to design self-assembled triplex structures from any other miR combination, or from other genetic materials, including antisense DNA or siRNA, to treat a range of diseases.
What do you think the future holds for microRNA delivery in cancer treatment?
Over 800 human miRNAs have been discovered to date, emphasizing the importance of these effector molecules in human body and adding a new dimension to our understanding of complex gene regulatory networks. Understanding and exploiting new platforms for controlling their expression, by inhibition or replacement therapies are of urgent need.
Consequently, nanotechnology and biomaterials have been gaining momentum in establishing solid knowledge in the development of new sensing and inhibition therapies using miRNAs in cancer. All the tools have been created.
Incredible advances have occurred in developed new nanovehicles capable of efficiently transporting miRNAs and antisense DNA or RNA oligonucleotides, especially in the systemic administration route, although to date, the majority of miRNA vehicles including liposome-based and some nanoparticle-associated have been found to accumulate primarily in the liver, spleen and kidney. For these reasons, miRNA local delivery via biomaterials scaffolding needs added support and focus.
Where can readers find more information?
Conde J, Oliva N, Atilano M, Song HS, Artzi N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nature Materials 15, 353–363 (2016) doi:10.1038/nmat4497
http://www.nature.com/nmat/journal/v15/n3/full/nmat4497.html
About microRNA delivery nanosystems:
About local delivery platforms in precision medicine
About other local delivery platforms we developed based on adhesive hydrogel scaffolds:
About Prof Artzi and Dr Conde
 Natalie Artzi is an Assistant Professor at Brigham and Women’s Hospital, Harvard Medical School. She is a principal Research Scientist at the Institute for Medical Engineering and Science at MIT, and is an Associate member of the Broad Institute of Harvard and MIT. Leveraging material science, chemistry, imaging and biology, Prof. Artzi’s lab is dedicated to designing smart material platforms and medical devices to improve human health.
Natalie Artzi is an Assistant Professor at Brigham and Women’s Hospital, Harvard Medical School. She is a principal Research Scientist at the Institute for Medical Engineering and Science at MIT, and is an Associate member of the Broad Institute of Harvard and MIT. Leveraging material science, chemistry, imaging and biology, Prof. Artzi’s lab is dedicated to designing smart material platforms and medical devices to improve human health.
Prof. Artzi pioneered basic understanding of tissue: biomaterial interactions and concepts learned have changed the way we view materials. Materials and devices are now being ‘personalized’ by considering specific tissue microenvironments that are altered in the face of disease.
Her multidisciplinary team works on developing materials for diagnosis and therapy, and exploit the toolkit available for material scientists to create multifaceted medical devices. She served as part of the scientific advisory board of Science Translational Medicine, serves as an associate editor of scientific journals and helps shape the future of biomaterials as part of the program committee of the American Society for Biomaterials.
More about the work conducted in Prof. Artzi’s laboratory can be found at: www.natalieartzi.com
 João Conde is currently a Postdoctoral Associate at the Massachusetts Institute of Technology, Institute for Medical Engineering and Science, Harvard–MIT Division for Health Sciences and Technology with a Marie Curie International Outgoing Fellowship for Career Development.
João Conde is currently a Postdoctoral Associate at the Massachusetts Institute of Technology, Institute for Medical Engineering and Science, Harvard–MIT Division for Health Sciences and Technology with a Marie Curie International Outgoing Fellowship for Career Development.
He obtained his PhD in Biotechnology in 2013 under the topic “Cancer Theranostics − Multifunctional gold nanoparticles for diagnostics and therapy” (part of the European project NanoScieE+ - NANOTRUCK), in NanoTheranostics group in the Research Centre for Human Molecular Genetics from Universidade Nova de Lisboa and in Biofunctional Nanoparticles and Surfaces Group from Institute of Nanoscience of Aragón.
His scientific interests are focused in (i) biofunctionalization of multifunctional metal nanoparticles with DNA/RNA, siRNA, drugs, dendrimers, fluorescent dyes, polymers, proteins/peptides and antibodies for (ii) cancer therapy and (iii) diagnostics; (iv) in vitro and in vivo applications of new nanomaterials and (v) nanoparticles toxicity/biocompatibility studies. He has authored more than 40 articles with almost 1000 citations and 2 US patents.