Today's medical science utilizes relatively simple anthropometric measures that describe the body, such as body mass index (BMI) and waist circumference. All of these measures are approximations of the body with the intention to characterize what's inside reflecting underlying phenomena that underpin the risk for different diseases.
Over the years, a lot of different techniques have been developed. Underwater weighing is basically our gold-standard technique for establishing the fat mass of the body. We have more modern versions of that, such as air displacement plethysmography or the Bod Pod technique, which accurately measure the volume of the body and body weight. From that, we can assess the total amount of fat in the body.
There are a number of techniques available for assessing the amount of lean tissue and fat accurately in the body. Depending on the technique, there are certain shortcomings, but I still think they serve a purpose for doing good phenotyping and establishing whether there is an excess or low level of fat in the body.
If you look at more modern techniques, there is the DEXA imaging technique, which is based on 2-D projections through the body with X-rays of multiple energy levels. This technique was developed for bone density measurement.
Over the last 5-10 years, we have seen this being used more and more for body composition assessment of both regional lean tissue volume measurement and regional fat volume measurement. It's a fairly cost effective technique, but with the strong limitation that it will always be based on projections through the body. That makes it impossible to assess the composition of a certain tissue and to tell whether the muscle tissue or the liver contains fat, for example. All these measurements will always be a model-based approximation of the amount of fat and are impossible to do accurately with DEXA.
To get to that step, you need to use the more modern imaging technologies that provide 3-D imaging of the body. Basically, CT and MRI are techniques that enable 3-D volumetric imaging. You can then image a muscle or a liver, for example, and assess the content in the pixel or voxel. This is much more specific and accurate in its description.
In this research area, CT- and MRI-based measures have been around for quite a long time. They have been used in different research settings to describe the liver fat or the visceral adipose tissue, which has some predictive power for adverse outcomes in different diseases.
This definitely adds a new level of accuracy in body composition assessment, not only establishing the total amount of fat and lean tissue in the body, but also describing where it is situated – whether it’s infiltrated in organs, as a sign of atrophy or metabolic disease and so on.
The focus of AMRA is to develop this further to make this a one-stop-shop, instead of having to use different technologies to assess different measures. We want to have one rapid exam that allows us to acquire most of the relevant information in a short time and at a reasonable cost.
I think the technology development we have done at AMRA is about how to take this further and to make it more industrialized, so the gains can be large while requiring a low level of effort.
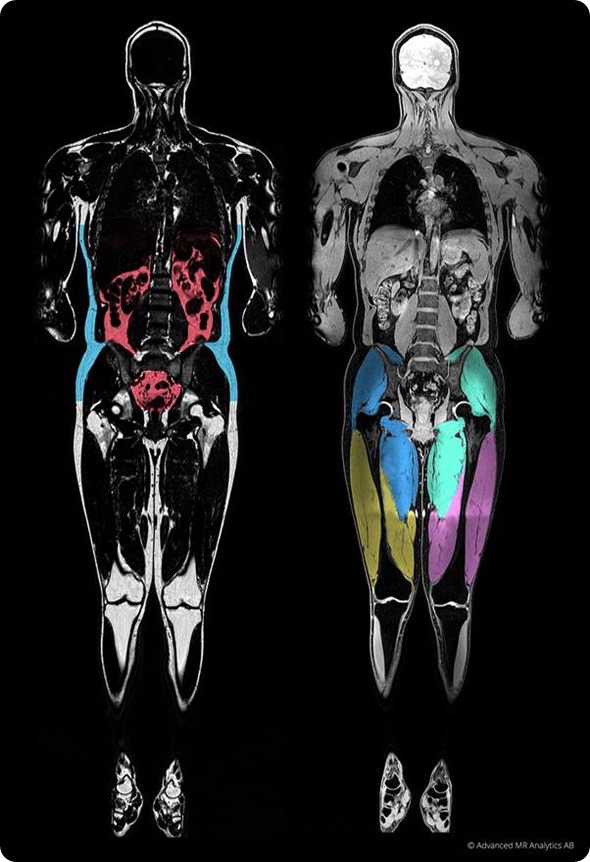
How can MR images be turned into body composition measurements?
The first major advantage of using MR images is that they do not use ionizing radiation, meaning we can perform an examination repeatedly without any associated health risks. Each scan provides a complete 3-dimensional description of the water and fat content in the body that allows us to characterize each voxel in the body separately.
Over recent years, MR imaging acquisition technologies have been developed that allow us to reconstruct images that have separate water and fat channels and are perfectly co-aligned, so-called Dixon imaging. Having the fat signal and the water signal in the bodies separated into two separate images is a major advantage when we want to characterize the composition of the tissue.
To take this further, a high level of standardization is needed and we apply this in different projects. To be able to call this body composition quantification, we need to do more or less the same when applying this in different situations because it's only if we do it in the same way that we can start to compare results from different sites.
Another aspect of this is that it needs to be rapid. Traditionally, an MR image examination took 45 minutes to one hour. We work on the philosophy that a MRI body composition scan would be a 5- or 10- minute scan – not more than that. That presents some challenges because you cannot use, for every part of the body, the most time consuming technique, correcting for all potential confounders in the underlying physics. That's also a major advantage of our technology - that it has extremely good performance, while also enabling a rapid scan.
The next step in turning an MR image into a body composition measurement is the image analysis of segmentation of tissue. We want to have localized measures and it is typically a time-consuming task for a radiologist to outline the image in different regions. What we are doing with our technology is reducing to a minimum the manual effort needed to extract the measures and therefore making the technique much more accessible.
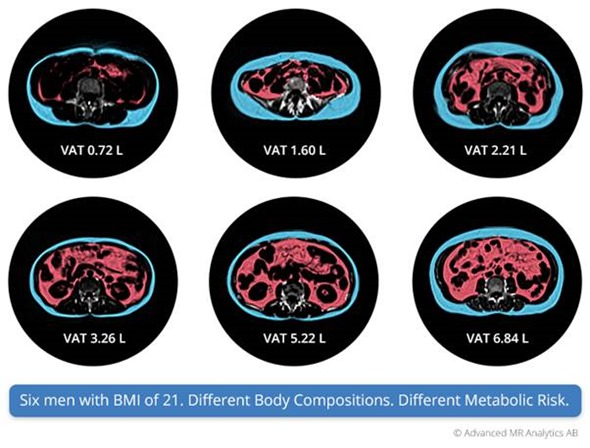
How does the accuracy compare to previous methods?
The major step is going from indirect to direct measurements. If you are only measuring from the outside, BMI, waist circumference, and so on, you cannot get an accurate description of the internal organs. Similarly, if you are using DEXA, you will have some decrease in accuracy with projection-based technology.
However, if you do the full MRI technology, you get more specific. If you look at what is considered to be the gold standard for localized organ volumetry today, it is manual segmentation of 3-D volumetric images from CT or MRI images.
It's difficult for us to prove higher accuracy than a manual technique. However, the major advantage we see when we apply the more highly optimized quantitative technique is that the reproducibility is increased, so the variability between different scans gets lower.
I don't think we can claim higher accuracy than the state-of-the-art today, but the big advantage comes with precision.
Could you please outline the research at Linköping University, Sweden that AMRA’s technology was developed from?
The technique originated from my and my colleague’s research at the Linkoping University in the Center for Medical Image Science and Visualization (CMIV). During my PhD work, I did a lot of research with quantitative MRI, both for brain and liver imaging. The aim was to develop methods that quantify the underlying physical properties of the tissue from an MRI scan.
The starting point for our joint research was being asked to help to develop an MRI measurement analysis for an endocrinologist who wanted to reproduce Morgan Spurlock's documentary “Super Size Me”.
Over the course of one month, Spurlock ate only McDonald’s food, ate as much as he could and did not exercise. He saw a tremendous health effect during this period. His liver blood values elevated, almost indicating that he was starting to develop liver disease, and he gained massive amounts of body fat.
We wanted to do a replication of the documentary in a more scientific context. We developed this imaging technology that allowed us to understand and quantify where in the body the fat was accumulated. We found that the typical gender characteristics in fat accumulation were such that males tend to store much more fat as visceral fat, while females tend to store it as subcutaneous fat.
From that project, we started to develop this technique into finer detail. We learned to quantify more fat compartments and later started to assess muscle tissue and muscle tissue composition.
A major focus of the research today is in projects such as the UK Biobank project, where we are currently working to apply the technique on a massive scale. We also want to explore interesting new compartments of quantification. One is focused on brown adipose tissue, which is an interesting, metabolically active organ that can burn energy rather than store energy like white adipose tissue does.
Another area is intramuscular fat, which seems to play a major role in several diseases, including muscular diseases, cardiovascular disease, and so on. Elevated levels of intramuscular fat seems to be a bad indicator. That’s where we are heading now with the research, but the research is currently quite well aligned with the work we are doing at AMRA.
What impact will the ability to generate body composition measurements from an MRI scan have on research?
I think we can divide it into two or three areas. First, I think for phenotyping, being able to do advanced, whole body imaging will enable us to characterize the subject in great detail. This is something that is important when it comes to precision medicine, where we need to have high reliability in every measure that goes into the model.
If we look at BMI on a population level, it has quite good characteristics in identifying that obesity is associated with high metabolic risk. However, when it comes to the individual level, it is not accurate or precise enough. We know that the measurements we provide offer a much more detailed phenotyping and characterization of whether patients in a clinical trial are high risk or low risk for metabolic syndrome and musculoskeletal disorders.
One obvious use for this is patient stratification in clinical trials. We can select the subject that has the highest probability of developing adverse outcomes or the highest probability of the drug having the greatest effect in the trial. We can then see if some patients should be on other therapies.
The other aspect is longitudinal follow up of patients and therapies. As our technology has high precision, it allows detailed characterization of the effects, but it also has an impact in that we can make better conclusions based on fewer subjects and the biomarkers that are more closely related to the physical metabolic syndrome like visceral fat and liver fat. Then we can get a better understanding of the intervention effects, rather than looking at just weight, which can be confounded by changes in muscle tissue, fat tissue and so on.
Another impact that I would like to highlight here is the rapidity of the scan. When the examination time of this goes down to 3-5 minutes, it can be included in many situations. In a cardiac imaging research trial, it is easy to add on a short scan and it provides you with a phenotyping, so that’s also how I see our technology – a one-stop-shop enabler of applying more detail in composition assessment.
Will this technology also be able to improve healthcare going forwards?
We believe so. At this stage, we are still in the research phase. One view we have about this is what we call "Redefining obesity."
We can compare it with cancer treatment. One hundred years ago, we saw cancer as a disease with a low probability of survival. Today we are using genotyping and phenotyping, and cancer is separated into several hundred types of diseases, each with an individual therapy. Most cancers today are treatable diseases.
If we look at obesity today, it is defined using BMI, which captures about 30% of the population. That is not a precise tool for characterization.
We need better tools to characterize the higher-risk patients, so that we can target more efficient therapies. We see our tool as important for identifying subjects with a high risk of developing cardiovascular events or adverse outcomes in diabetes and so on. However, we need to acquire a lot of information in order to reach this stage. That's why large-scale imaging studies such as UK Biobank are important, because that's the platform where we can acquire longitudinal health outcome data to prove the value of the technology.
How do the total body composition analysis costs of AMRA’s technology compare to other methods?
To understand this, you need to separate it into two different costs. First, there are the standard costs. MRI is traditionally seen as an expensive modality. However, using our technology, the scan time is low, which reduces the costs. You are able to scan more subjects per hour and, also, we don't need a manual reading of the images. The time taken by radiologists accounts for another large fraction of the MRI costs.
We have decreased the costs on both sides. Considering the cost of the analysis and the total cost of the scan, I think the advantage of our technology is that we have a one-stop-shop that provides you with the most relevant body composition assessment, without having to use several scans with different modalities.
Even if MRI is a reasonably expensive device to use, there is the amount of information you obtain. It can also be combined with all other conventional MRI imaging – cardiac imaging, liver imaging, and so on, that you perform in the same set up. So, it is nicely packaged into the workflow of the MRI scanner.
What do you think the future holds for body composition analysis and how do AMRA plan to add to this?
Traditional body composition measurement describes the complete body – how much is fat, how much is water, how much is muscles and so on. However, as we develop this quantitative imaging technology, we get more and more detail and a quantitative scan can assess smaller muscle groups related to musculoskeletal disorders or it can assess pancreatic fat or liver fat. It allows more focal characterization.
The border between diagnostic imaging and body composition imaging will not be that clear anymore. When we reach that stage, we will see a major advantage of the quantitative imaging technology, because with an automated technology that extracts important information about every subject's scan in great detail, we can accumulate data obtained from the data analysis nicely, and this can be combined with healthcare and health outcome registries to understand the relationship between different characteristics in the body and health outcome.
If we don't take that step into the quantitative imaging, we will have great difficulty making that transition with traditional radiology, with the manual interpretation of images that is not well-suited to collecting large-scale data analysis.
Where can readers find more information?
http://amra.se/
About Olof Dahlqvist Leinhard
Olof Dahlqvist Leinhard is AMRA's Chief Technology Officer responsible for technical vision, for leading the execution of technology platforms and partnership strategies, and for overseeing all technology research and product development. Olof is also a Founder and Member of the Board.
Since 2012, Olof has performed as a Junior University Lecturer in Magnetic Resonance (MR) Physics at Linköping University (LiU), within the Department of Medicine and Health (IMH) / Division of Radiological Sciences (RAD). His position has included the PhD supervision of five PhD candidates, as well as the student supervision of over 20 individuals within MR Physics and Radiology. From 2010 to 2014, Olof also served as the Director of Doctoral Studies at the Center for Medical Image Science and Visualization (CMIV).
Renowned within the field of MR Physics, Olof has over 35 peer-reviewed journal and conference articles, as well as over 60 peer-reviewed conference abstracts to his name. Olof earned a PhD in Medical Radiation Physics at LiU in 2010, following a Licentiate Degree in Medicine in 2008, and a Master of Science in Physics in 2004.
Olof currently holds the following assignments:
- Member of the Scientific Council of CMIV
- Member of the Board of SMIL, a network for SMEs in Linköping
- Examiner / Course Coordinator for Molecular Imaging 7.5 Hp, Advanced Level
- Member of the International Society for Magnetic Resonance in Medicine
- Reviewer for European Radiology and European Journal of Radiology