We control their electrical activity. Cardiac cells are capable of producing and transmitting electric signals through changes in a cell membrane potential.
Propagation of this electric signal, as “excitation wave” through the cardiac tissue triggers cardiac cell contraction.
The excitation waves in the heart are very important to keep coordinated contraction of the cardiac cells, so that heart can fulfill its main function: to pump blood.
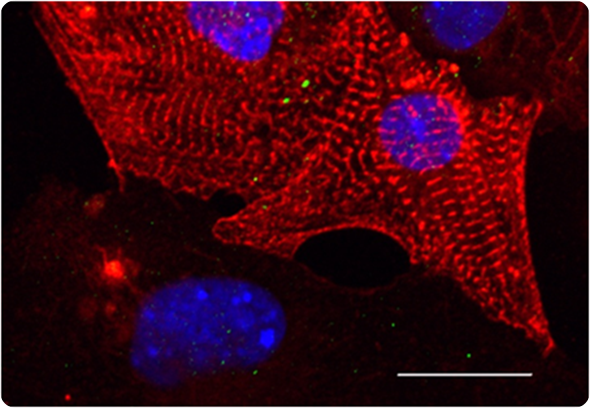
Micrograph of heart muscle cells (cardiomyocytes)
Images credit: authors of the study
What are arrhythmias and how did you create “arrhythmia in vitro”?
When a heart loses its normal rhythm – it is called arrhythmia. The most dangerous arrhythmia is tachyarrhythmia caused by the very special sources of excitation: rotating waves.
Cardiologists call them “re-entry”; physicists often refer to them as “spiral waves” or “rotating waves”. They can subdue normal heart rate and what is even worse, they may multiply, independently control activity of different parts of the heart, eventually completely destroying the orchestrated functioning of cardiac cells.
We study rotating waves of electrical activity in the tissue culture: in vitro.
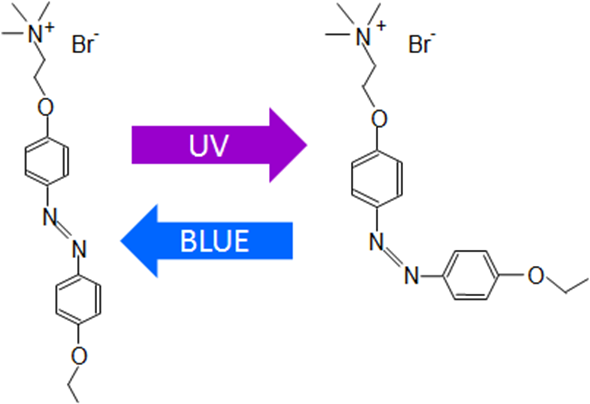
Diagram of the azoTAB isomerization
Images credit: authors of the study
How did you teach the azoTAB molecules to control cardiomyocytes?
The quaternary ammonium group in the molecule does the job to control the electrical activity of the cardiac cell. However, it is not our invention: our colleagues used this molecule to change the permeability of the lipid bi-layer, we just got the idea that it should work in the live cell.
How much is currently known about the way the different forms of azoTAB affect cardiomyocytes?
Our recent paper in PLOS ONE shows that the main mechanism of azoTAB action is modulating the activity of transmembrane ion channels: in the trans- form it blocks sodium and calcium channels, in the cis- form it does nothing.
In what ways will this study help scientists to better understand the mechanisms of the heart?
More precisely, it helps us understand the mechanisms of cardiac arrhythmia. Since azoTAB can reversibly change the excitation of the cardiac cells, we are able to use it for modeling different conditions in the experiments with the cultured heart tissue.
We are projecting a computer generated pattern on the layer of cardiac cells. It gives us an opportunity to “draw” on the cardiac tissue various conducting ways for the excitation waves, different obstacles and also, make this pattern time-dependent. Thus, we can study the conditions for re-entry origination and the ways to extinguish them.
Could this technique have clinical implications moving forwards?
I hope so, yes. Unfortunately, azobenzens are quite toxic, so we are searching for different substances possessing similar features with much less toxicity. Now we have promising results with the substances called stilbens.
What further research is needed before this technique could be used in clinical practice?
We need to find less toxic substance instead of azobenzene. As I mentioned, we already have one candidate, but it is not published yet.
In addition to arrhythmias could this research potentially help with any other cardiac pathologies?
We are focused on the arrhythmia based on the pathological excitation propagation.
What are the next steps in your research?
To show that stilben analogue of azoTAB (we call it c-TAB) works well not only with the cultured cardiac cells but also may be used in the animal experiments.
Also, since azoTAB is capable to control the activity of cardiac membrane ion channels, it is most probably, could be used for controlling nerve excitation.
Some preliminary data strongly indicate that azoTAB ccan control generation and propagation of nerve impulses. In that case, it might open the way for developing very precise light-activated easily reversible anaesthesia.
Where can readers find more information?
http://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0152018
About Prof. Konstantin Agladze
In 1978 he graduated from the Moscow Institute of Physics and Technology. He worked at the Institute of Biological Physics of the USSR Academy of Sciences, then at the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences.
Since 2000, Agladze worked in leading research centers in the US and Japan (Florida State University, University of North Texas Health Science Center, Emory University School of Medicine, Atlanta, George Washington University, Washington, DC; Kyoto University, Japan).
Agladze is one of the winners of the first contest of the Russian Government “megagrants program”, 2010. Since 2013 he is a Head of the Laboratory of Biophysics of Excitable Systems, Moscow Institute of Physics and Technology.