PGS, Preimplantation Genetic Screening, is a genetic test that analyses biopsied cells from embryos produced by in vitro fertilization (IVF) techniques.
PGS determines whether the embryos are chromosomally normal (euploid) or not (aneuploid), thus giving the chance to transfer chromosomally normal embryos that are more apt to successfully implant and develop into a pregnancy.
Widely accepted indications for PGS are those in which there is an increased risk for the couple of generating aneuploid embryos: advanced maternal age; history of recurrent miscarriages or pregnancies/children with chromosomal abnormalities; recurrent IVF failure (>2); chromosomal translocation carriers.
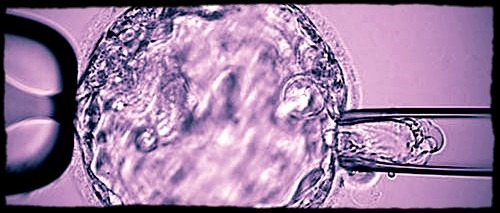
Biopsy procedure of a blastocyst (day-5 embryo) for subsequent preimplantation genetic testing
What is next generation sequencing (NGS) and why did Bioarray develop PGS using NGS in 2014?
In short NGS is a term that encompasses a number of proprietary DNA sequencing technologies that superseded capillary sequencing in terms of speed and cost thanks to its high-throughput, especially suitable when large amounts of DNA are analyzed.
Bioarray had a previous extended experience using NGS in the field of medical genetics, for prenatal and postnatal diagnosis, but for PGS in 2014 we were using microarray CGH (comparative genomic hybridization), the most advanced established PGS 24-chromosome technology at the time.
However CGH was an expensive technique not affordable for all the couples undergoing an IVF cycle. For this reason, we decided to develop a new protocol based on NGS for chromosome screening.
What impact has NGS had on PGS so far?
As for clinical results, with one of our partner fertility clinics alone (IVF-Spain), we have provided PGS to more than hundred European couples, with the first born baby screened by this method in September 2015.
To date an overall of 64 born babies and 53 ongoing pregnancies have been achieved. This and the rest of our partners report similar or better clinical outcome than with CGH.
From a technical perspective our comparative validation of NGS vs CGH resulted in 100% correspondence in aneuploidy detection but with higher detection power for smaller chromosomal gains/losses using NGS than with CGH.
What is preimplantation genetic diagnosis (PGD) and how has this been provided by NGS?
PGD, Preimplantation Genetic Diagnosis, on the other hand, is intended for couples who know that one (dominant disease) or the two of them (recessive disease) are carrying a specific disease-causing mutation.
PGD detects the presence or absence of the mutation in the embryos in order to prevent the transmission of the disease (e.g. cystic fibrosis) by transferring only those without mutation.
PGD relies on the direct detection of the mutation but also on an indirect analysis of linkage polymorphisms that help us be sure that no allele drop-out occurred, so we are “seeing” both alleles.
Before NGS, PGD required a second technique since STR (short tandem repeats) were typically used as linkage markers. Now NGS can be used alone, since it uses SNPs (single nucleotide polymorphisms) as linkage markers. Plus it also allows merging PGD with PGS in a single procedure.
How did Bioarray integrate PGD setup and PGS?
A double approach is combined in the same workflow: an ultra-low coverage is used for the copy number variation analysis (PGS) in the embryos whereas high coverage is used to sequence the region of interest where the mutation (PGD) is, both in the embryos and the couple (plus more family members if necessary). Also including a tag-SNP method for the analysis of the mentioned linkage markers.
What do you think the future holds for PGS?
We are working to obtain extra valuable information from NGS-PGS in addition to the main chromosomal copy number status.
Detection of mosaicism i.e. different chromosomal statuses among the biopsied cells (trophoblasts) that we analyze as whole (trophectoderm), and quantification of mitochondrial DNA which may be representative of the embryo’s potential to implant and further develop, will add additional input to select those embryos more likely to implant. By this way we expect to reduce even more the time to achieve a pregnancy in IVF cycles.
Also the drop of the costs will make this technique widely accessible to all couples.
What is Bioarray’s vision?
Bioarray aspires to keep on the frontline of reproductive genetics after having pioneered the application of NGS to PGS and PGD.
Our commitment is to provide our partner IVF Clinics and their patients with the most cutting-edge techniques and for this purpose we benefit from our great specialization in molecular technologies which we also extensively use in the more wide and complex field of medical genetics.
Where can readers find more information?
About Dr Luis Alcaraz
Scientific and Lab Director as well as cofounder of Bioarray.
Biologist and researcher in the field of proteomics and genomics, using high-throughput techniques, with a comprehensive experience in bioinformatics.
Former positions in several public and private research centers and companies.