Paxman Coolers Limited announced today that The U.S. Food and Drug Administration (FDA) has cleared the company to market the Paxman Scalp Cooling System, a scalp cooling technology that was developed by a British family to reduce hair loss in breast cancer patients undergoing chemotherapy.

The concept behind the pioneering Paxman Scalp Cooling System came when the mother of four, Sue Paxman, experienced first-hand the trauma of chemotherapy induced hair loss.
The Company has since been on a personal journey to ensure Sue’s legacy lives on by helping women around the globe minimize chemotherapy-induced hair loss and contribute to their quality of life.
“Hair loss is consistently ranked in the top five most distressing cancer chemotherapy side effects,” explains Richard Paxman, CEO at Paxman.
“Like my mum, many people find hair loss to be extremely traumatic. It is estimated that 8% of patients actually refuse chemotherapy because they do not want to lose their hair. After experiencing this first hand, we have been determined to change this, and help minimize hair loss in women who are undergoing chemotherapy for breast cancer, positively contributing to their overall health and recovery.”
As part of the FDA clearance process, the Paxman scalp cooler was used in the first-ever randomized clinical trial to evaluate modern scalp cooling, which took place at a number of sites including Baylor College of Medicine, Memorial Sloane Kettering, the U.S. Oncology Network, Cleveland Clinic & Summit Medical Group - MD Anderson Cancer Center.
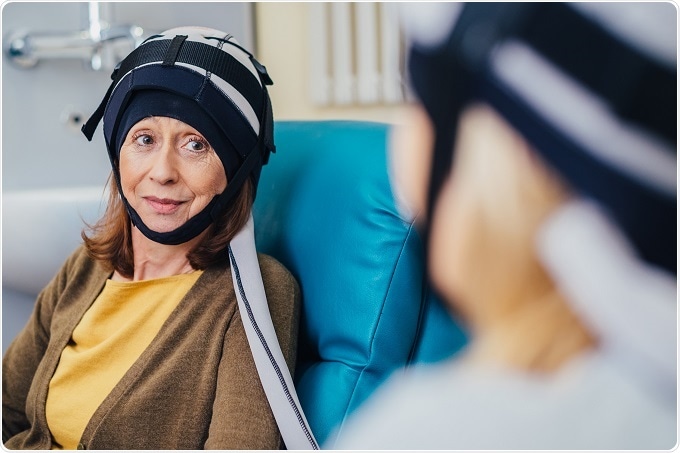
The multi-center prospective study, which involved 186 women across New Jersey, New York, Texas, and Ohio, revealed that the cold cap preserved hair in more than 50% of the women who used it.
Speaking about results lead researcher Dr. Julie Nangia, MD, Assistant Professor of Medicine at Baylor College of Medicine, Houston, Texas, USA added: “The Paxman Hair Loss Prevention System is a safe and effective method for reducing hair loss in women being treated with chemotherapy for breast cancer, especially for those on taxane-based regimens.”
“Hopefully in five years from now, we will consider scalp cooling part of routine practice, the same way that we can see an IV-pump with an IV-pole as part of the regular equipment you would expect in an infusion suite. It’s important that people undergoing chemotherapy understand what scalp cooling is and that it is an option available to them if they want to prevent hair loss.”
Over the next 12 months, Paxman plans to install 250 systems across the U.S. and will be working with a large number of cancer centers and large community oncology groups to roll out their scalp cooling systems.
The Company aims to revolutionize the current landscape of scalp cooling by ensuring that affordability is at the center of the treatment giving more patient choice.
This involves launching a single patient use personal cap model, which will be loaded with scalp cooling credits depending on the number of chemotherapy infusions they are having.
Patients using scalp cooling treatment will only pay for their own cap and the treatment they use.
Richard added: “The USA is the largest healthcare market in the world with over 1.6 million diagnoses of cancer each year. We have spent six years conducting a comprehensive multi-center randomized clinical trial to ensure our data is as robust as possible. This has been a significant investment for us, but we are incredibly excited to be able to offer scalp cooling to US patients giving them a choice to maintain some control during treatment as we see in the UK and other parts of the world.”
How scalp cooling works
Chemotherapy works by targeting all rapidly dividing cells in the body. Hair is the second fastest dividing cell, and this is the reason why many chemotherapy drugs cause alopecia. The hair follicles in the growth phase are attacked, resulting in hair loss approximately two weeks after the commencement of the chemotherapy treatment.
The damage that chemotherapy causes to the hair follicle can be alleviated by using scalp cooling, also known as the 'cold cap.'
 Scalp cooling works by reducing the temperature of the scalp by a few degrees immediately before, during and after the administration of chemotherapy. There are a number of mechanisms by which cooling protects the hair follicles: vasoconstriction reduces the amount of chemotherapy that reaches the hair follicles, reduced drug diffusion through the cell membrane, decreased cell division, reduced active transport mechanisms, and a general reduction in metabolic activity.
Scalp cooling works by reducing the temperature of the scalp by a few degrees immediately before, during and after the administration of chemotherapy. There are a number of mechanisms by which cooling protects the hair follicles: vasoconstriction reduces the amount of chemotherapy that reaches the hair follicles, reduced drug diffusion through the cell membrane, decreased cell division, reduced active transport mechanisms, and a general reduction in metabolic activity.
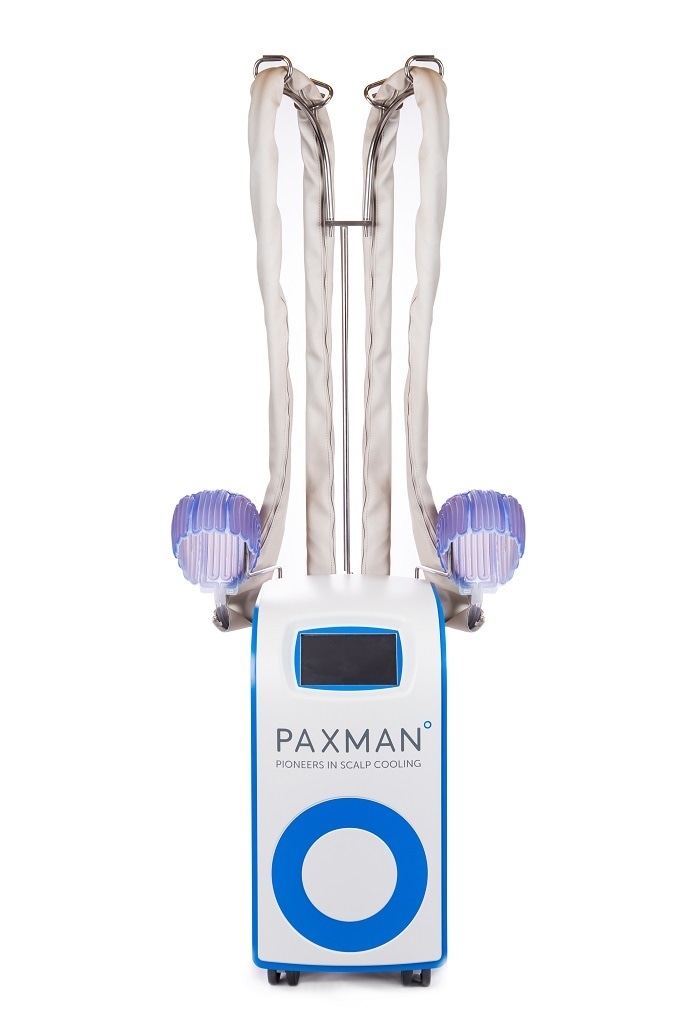
Paxman Scalp Cooling technology, with over 2500 systems in hospitals, clinics, and treatment centers around the world, is made from lightweight, biocompatible silicone. The scalp cooling cap is soft and flexible, providing a snug yet comfortable fit during treatment.
The Paxman Cooling Cap molds to all head shapes and sizes. The liquid coolant passes through the cap, extracting heat from the patient's scalp, ensuring an even, constant temperature is maintained to minimize hair loss.
The prevention of hair loss represents an important challenge in oncology, which affects over four million patients annually. By 2030 it is estimated it will affect over 6.7 million patients every year.