The U.S. Food and Drug Administration yesterday approved the first magnetic resonance imaging (MRI) device that can be specifically used in neonates or newborn babies. These MRI devices would be used for imaging of the brains and head in neonates who are admitted to the neonatal intensive care units (NICU) where they are necessary.
MRI or magnetic resonance imaging is a form of radiological imaging technique where the scanners use strong magnetic fields to produce the images of the inner structures. The protons in fat and water molecules in the body help form an image of the insides of the body. MRI brain is indicated in several conditions some of which are life threatening and MRI can help detect these conditions. Newborns admitted to the NICU may also require an MRI scan as part of their investigations whereby important decisions may be made.
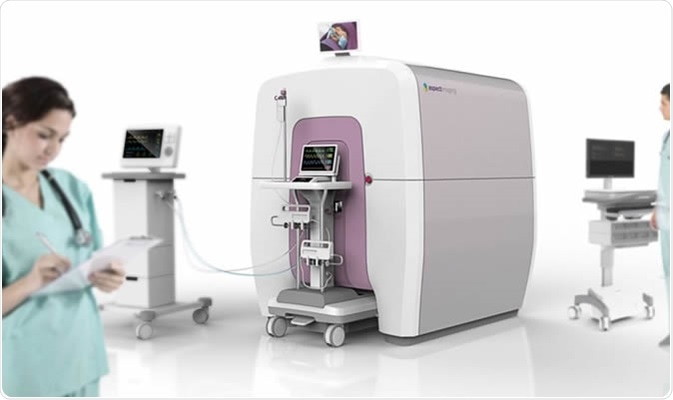
Embrace Neonatal MRI System. Image Credit: Aspect Imaging
Vasum Peiris, chief medical officer for pediatrics and special populations at FDA’s Center for Devices and Radiological Health, said that till date whenever MRI brain was indicated for neonates in the NICU, the babies had to be taken to the MRI units. Traditional MRI images thus were possible but the challenges of moving a sick newborn out of the NICU to the MRI suite would often be a challenge. This new system is exclusively for neonates and now it would be safer and more convenient for imaging the brains of these ill newborns. He said that this approval ensures “safer imaging for this vulnerable patient population”.
The newly approved MRI system for neonates is called the Embrace Neonatal MRI System. It can measure the heads of the neonates only. Babies with a head circumference up to 38 centimeters and weight between 1 and 4.5 kilograms can be imaged in this machine. Larger babies and children cannot be imaged in this machine. Those with metallic implants within their bodies also cannot be imaged using this system much like traditional MRI machines.
To make the transition from the NICU cots to the MRI machine comfortable as well as less risky for the ill newborn, this new system also has a temperature-controlled incubator inside the MRI system. This makes the baby comfortable and ensures that the baby moves less. In case of an emergency where the baby needs immediate attention, the baby can be removed from the machine within 30 seconds and resuscitated. The system can be placed inside a NICU safely.
The machine is safe with no risk of radiation release and thus need not be in a radiation-safe room. Further the Embrace Neonatal MRI System is completely enclosed and thus it does not mandate what is around it. Most MRI machines cannot allow metallic elements around or within them. The objects that can be allowed inside traditional MRI machines are termed “MR safe” and those less safe are termed “MR conditional”.
The approval of the machine comes from non-clinical testing and the system has not yet been tried on actual neonates. This was done to avoid putting the vulnerable patients at risk. Electrical and mechanical safety of the system was thoroughly examined before approval. The Embrace Neonatal MRI System clearance was granted to Aspect Imaging Ltd. by the FDA.