An interview with Dr. Robert Switzer, President and Chief Scientific Officer of NeuroScience Associates (NSA), conducted by April Cashin-Garbutt, MA (Cantab)
Can you please outline the vision behind NeuroScience Associates (NSA)?
Leveraging the mass production benefit that MultiBrain® technology brings to neurohistology, NSA can accelerate the R&D preclinical and safety assessment processes many fold and perform them less expensively. This results in faster times for a potential drug to move from R&D to clinical trials and sooner for use in people.
Additionally, utilizing some of the same principles, our proprietary Large Format™ technology enables us to embed and section an entire human brain hemisphere intact. This not only speeds up processing, but dramatically improves visualization of the entire hemisphere, vs the standard practice of viewing small 1-2cm slabs.
How is NSA able to perform neurohistology up to 40X faster than traditional methods?
Traditional histologic methods section one brain at a time using paraffin embedded tissue or freeze-sectioning with cryostats. With MultiBrain® technology we embed up to 40 mouse brain hemispheres or 40 spinal cords into one MultiBrain® gelatin block. The block is freeze-sectioned with all of the cut sections collected into an array of containers filled with ‘antigen preserve’ solution.

Image credit: Huron Digital Pathology.
The resulting ‘sheets’ of sections are stained, usually ‘free floating’ (not mounted on slides yet) which allows all of the sections from different brains contained in the sheets to be exposed to identical staining conditions. This is extremely important in interpreting staining differences across different brains.
How large is the tissue you are processing?
A wide range of sizes of tissue can be accommodated by our technology: mouse spinal cords to human brain hemispheres. Of course, only one human brain hemisphere can be embedded in one block! Most of the large tissues we process are from the brains of non-human primates, dogs, sheep and pigs. The larger brains are important in that they mimic more closely the human brain.
What are the challenges with sectioning such large tissue?
The challenges of sectioning the Large Format™ tissues are not significant compared to a block of mouse or rat brains. Since the tissue is being frozen and then sectioned, it’s important that the tissue is adequately treated with so called ‘cryo protect’ solutions, which retard the size of ice crystals that form when the block of tissue is frozen.
Such crystals, if allowed to grow, would create large tears in the tissue and ruin the anatomy. Traditionally, sucrose (20-30%) has been used to saturate the tissue, but for the last 30 years we have used a 20% glycerol solution that yields superior results.
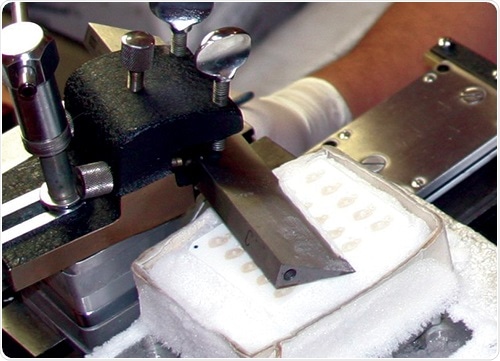
Image credit: Huron Digital Pathology.
What does the multiple embedding process involve?
MultiBrain® technology allows for many brains and/or spinal cords to be embedded in a single gelatin matrix block. From that point forward, the tissues remain together for all staining procedures.
What impact has the TissueScope LE120 whole slide scanner from Huron Digital Pathology had on your workflow? Did anything surprise you when you started scanning?
The Tissue Scope LE120 has allowed us to meet a growing demand from scientists for high quality digital images of the stained sections. Up until a few years ago, we only provided stained slides of brain or spinal cord tissues. At the same time that the demand grew for digital images of the brain and cord sections, the demand for providing image analysis skyrocketed. Of course, in order to perform the analyses, digital images are required.

Image credit: Huron Digital Pathology.
The LE120 allowed us leverage MultiBrain® processing because Huron’s software engineers designed programs that could capture images of individual brain sections from one MultiBrain® sheet and group them.
The common practice with other image capture systems on the market is to capture the whole slide (holding up to 40 different specimens) and leave it to the user to pluck out the individual sample images. That calls for human involvement which is costly and time consuming. With Huron’s system we continue to accelerate the process of extracting data from our clients’ experimental tissues.
Our biggest surprise when we first began using the LE120 was how easy it was to use, especially with the accommodations that the software engineers provided to meet our rather unique requirements.
Can you please explain how NSA is involved in large format technology that allows the sectioning of large intact brains continuously without the need to subdivide into slabs or cubes?
The capability to section large tissues is governed by the microtomes used to cut the sections. Most microtomes are designed for tissue embedded in paraffin blocks. Tissues are usually small samples so they are embedded in small blocks of paraffin. Since most microtomes available can only accommodate a ‘postage stamp size’ block, tissues larger than that must be subdivided.
For brain studies of animals larger than rats, such subdividing makes analysis quite impossible to assess the anatomical context of a full, intact brain. The microtomes we used were designed in the early 1900s—The AO860 microtome is our workhorse.
How does Huron help in terms of digitizing the large finished slides?
It appears that from the onset of Huron’s evolution in designing digital capture instruments that image of large tissue such as a full human brain was their intent. Huron’s designs allow standard 1x3” slides to be digitized but can also digitize slides as large as 6x8”- a whole cross section of human brain.
What was your motivation for moving to a digital workflow and what benefits are you seeing from the transitioning to digital?
This has been not so much ‘transitioning to digital’ as it is to include it as part of our service package—along with image analysis. The speed and results of the newer image analysis tools are behind most of the demand in digitization.
How are you dealing with the large amounts of data generated from scanning large tissue?
The file sizes of the digital images are huge—measured in gigabytes versus megabytes in conventional microscope cameras. NSA does not currently store or provide a server of the images to clients. Rather, we ship a hard drive containing the images captured on behalf of the client. The original image data captured at NSA is deleted sometime after the client has received the hard drive.
The large files sizes are posing a challenge to many of the current software programs which have not grown to keep pace with the new capabilities of full-section digital capture. There are work-arounds, but the demand is there so it should improve in the near future—we hope!
Do you store the files locally or on the cloud?
Files are temporarily stored locally. They are sent physically to the client.
Can MultiBrain® principles be applied to a variety of tissue types, orientations and numbers?
MultiBrain® technology is quite versatile and can accommodate many variations on a theme of orientations and numbers. Tissue types are restricted by the physical integrity of the tissue in the sectioned state. We confine our service to brain and spinal cords.
Other tissues, such as spleen, liver, kidney, dorsal root ganglia have occasionally been successfully processed because when ‘fixed’ by formaldehyde the sections stay intact and don’t disintegrate as would happen with thymus, adipose, or pancreatic tissue, for example.
What do you think the future holds for neurohistology?
Neurohistology will always be needed because no matter what other kind of in vivo imaging capabilities may be developed, one must always, eventually, look to see what is happening in the tissue itself.
NeuroScience Associates (NSA) Overview 2017
What is next on the horizon for NSA?
Image analysis capabilities is the biggest growth area that I see. Being able to assign some kind of numerical data point to the stained structures/features on slides is in high demand. The range of possibilities is almost endless because it all comes down to what the investigator wants to see or compare.
The next area posing the biggest challenge is being able to mine the data that is generated. This is where the informatics folks will find lots of work!
Where can readers find more information?
Please go to our website: NSALabs.com and if you can’t find what you are looking for there, please call or email us: [email protected]. We enjoy such inquiries and it helps us stay current and to be ‘ahead of the curve’ in identifying unmet needs.
About Dr. Robert Switzer

Dr. Robert C. Switzer, III has over thirty-five years of experience in the neuroscience field. Immediately prior to founding NSA, he was the Director of the R. H. Cole Neuroscience Lab at the University of Tennessee which focuses on research related to Parkinson’s and Alzheimer’s disease. Dr. Switzer also has held positions at the National Institute of Mental Health and the Comparative Animal Research Lab in Oak Ridge Tennessee.
Dr. Switzer has authored more than forty manuscripts in peer reviewed journals, published over fifty scientific meeting abstracts and contributed to seven books. In addition, Dr. Switzer is the inventor of NSA’s MultiBrain® technology and was awarded a patent for silver staining techniques used to detect Alzheimer’s disease pathology.
Dr. Switzer graduated from Michigan State University with a B.S. in Physics and Astronomy and then joined the Biophysics department under Dr. Barnett Rosenberg to study olfactory transduction mechanisms. Later, the intrigue of comparative neuroanatomy attracted him to the laboratory of Dr. John I. Johnson under whom he obtained his M.S. and Ph.D. in Biophysics.
As a post-doctoral fellow under the tutelage of Drs. Lennart Heimer and Jose deOlmos at the University of Virginia he learned neurohistologic silver staining techniques for identifying neuronal pathways in the brain. As an NIH Staff Fellow, he evolved the application of silver staining methods to specifically detect neurotoxic effects. The neurotoxic effects of alcohol and high pressure oxygen were among his first applications of the silver staining methods for this purpose.
Among Dr. Switzer’s most significant “technical” contributions to neuroscience are the invention of MultiBrain® technology, the Campbell-Switzer Alzheimer’s stain and also the development of the first silver stain for proteins in 2-D gels. Some highlights of his “scientific” contributions to neuroscience include papers published on the neurotoxic effects of alcohol, a neuroanatomical trait as a phylogenic discriminator, absence of mitral cells in monotremes, and the locus of ventral globus pallidus as defined by ferric iron.