A device serving as a new treatment option for moderate to severe central sleep apnea has been approved for use by the U.S. Food and Drug Administration (FDA).
 Credit: Africa Studio/Shutterstock.com
Credit: Africa Studio/Shutterstock.com
The implantable device stimulates a nerve in the chest that carries signals to the diaphragm telling it to move and induce breathing.
Tina Kiang from the FDA’s Center for Devices and Radiological Health says patients with central sleep apnea should talk to their healthcare providers about the health risks and benefits of using this new treatment option over others, which include medication, the use of positive airway pressure devices and surgery.
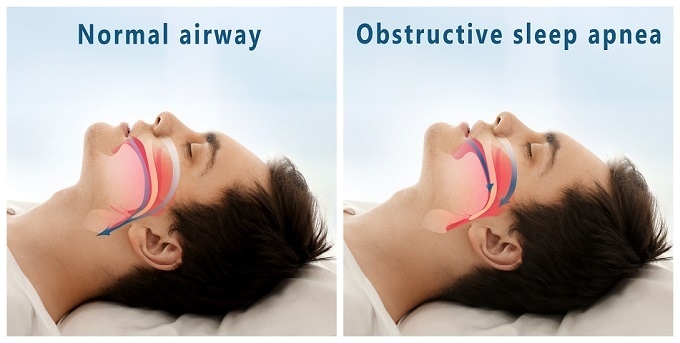
Sleep apnea is a condition characterized by pauses in respiration during sleep that can last anything from at least 10 seconds, up to minutes. This happens because the brain fails to send signals, via the phrenic nerve, to the diaphragm to stimulate breathing. The National Institute of Health’s National Center on Sleep Disorders warns that this can lead to an increased risk of various health problems including high blood pressure, heart attack, heart failure, stroke, diabetes and obesity.
The device is made up of a battery pack that is surgically implanted beneath the skin and small wires that are inserted into blood vessels close to the phrenic nerve. It monitors a person’s breathing signals while they are asleep and, if needed, triggers the phrenic nerve to send signals to the diaphragm so that normal respiration can be restored.
To assess the effectiveness of the device, the FDA evaluated data available for 141 patients who had the frequency and severity of their apnea events per hour monitored using a measure called the Apnea−Hypopnea Index (AHI). After six months, AHI was reduced by at least half in 51% of those who had the device implanted, compared with a reduction of only 11% among patients who did not have it implanted.
Adverse events most commonly reported included concomitant device interaction, infection at the site of implantation and swelling and local tissue damage or pocket erosion. The instrument must not be used to treat individuals who have an active infection or those who are known to need magnetic resonance imaging. It is also not intended as a treatment option for obstructive sleep apnea, a condition where breathing is compromised by partial or complete blockage of the upper airway.