Apr 10 2018
With at least two in three Australians diagnosed with skin cancer before the age of 70, monitoring moles and skin is vital in detecting skin cancer early for a generation of people who spent much of their upbringing in the sun (often with little to no protection).
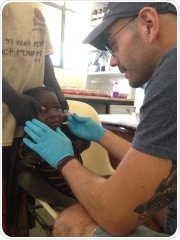
To meet the massive unmet need in this area, Research Professor at UniSA’s Future Industries Institute, Professor Tarl Prow led the team that has created a new needle device that will revolutionize how doctors test patients for rashes and diseases.
The microbiopsy device is a small needle that can take 100 to 200 cells to test for skin cancer and other diseases avoiding the need to take a 2cm to 3cm piece of tissue which results in some scarring.
Dr Prow says the device can take skin samples much more easily than a traditional biopsy and is easy and painless to use on children.
“The innovation for this device comes from the idea of a diabetic test. We can easily take small skin samples which makes it much easier to use on children and allows us to take multiple samples over time to monitor a patient’s situation,” Prof Prow says.
“Given a standard biopsy is a significant procedure, we wanted to help make that process easier.”
The device has already proved a possible game changer in underprivileged countries with doctors from Jerusalem’s Hebrew University already taking 502 units to Africa to use in the field.
“With many parasitic diseases in Africa, where people don’t have access to a hospital, researchers at Hebrew University undertook a study in northern Ethiopia testing hundreds of children and adults using the device,” he says.
With skin cancer a more prevalent problem in Australia, doctors can spend up a significant amount of time checking a patient’s skin (including moles and pink spots) and Dr Prow and his team are working on developing cancer biomarkers, so the device can take samples and evaluate (and reveal) the results instantly.
With some clinical trials (unrelated to skin cancer) in the final stages, the team is monitoring feedback with thousands of devices already sold worldwide and is patented in the US, Australia and Europe.
“We are planning to test the efficacy and safety for microbiopsy in skin cancer diagnosis that will start in early 2019 so some patients may be asked to be a part of this study. We hope to launch the approved diagnostic test in 2023,” Dr Prow says.
Source: http://unisa.edu.au/Media-Centre/Releases/2018/-New-needle-device-to-revolutionise-biopsies-and-reduce-scarring-/