Last week the United States Food and Drug Administration (FDA) announced its approval of the digital birth control app to be sold in the US. The app is called “Natural Cycles” and is one of the first medical devices of this kind to receive the FDA nod.
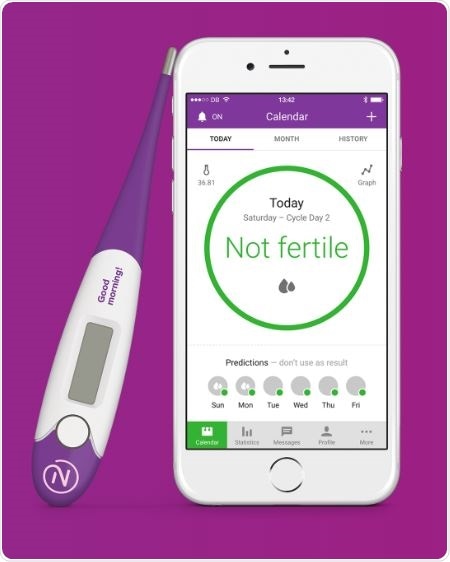
Digital birth control
This new app is a digital version of the natural method of birth control or rhythm control. The app uses past menstrual history to predict the course of the cycle telling a couple when the woman is most likely to be fertile. The app’s makers are a Sweden-based company Natural Choices and it uses changes of the body temperature of the woman to predict ovulation dates. The body temperature increases by one degree or less in most women when their ovaries release the ova or when they are ovulating. Having unprotected sex during that time can increase the chances of getting pregnant.
To detect such small changes in temperatures the app is connected to a digital thermometer that can measure the body temperature with an accuracy of two digits after the decimal. The app purchase includes a highly sensitive digital thermometer. For couples trying to conceive the app can mark certain days of the calendar as green and red indicating fertile days and non-fertile days respectively. This app can also help couple prevent unwanted pregnancy by having protected sex during the green marked days.
The FDA has a de novo premarket review pathway that was utilized to clear this app for marketing. This allowed a faster review process for the app. This de novo pathway is used for low- to moderate-risk medical devices approved for public use. According to the FDA statements the app is good for preventing pregnancy when compared to other contraceptive methods.
For the approval the FDA was provided with clinical studies using information from 15,570 women. These women were using the app for at least eight months. The makers found that the app, when used correctly had a failure rate at preventing pregnancy at 1.8 percent. This translates into less than two women getting pregnant per year if they used this app alone as a contraceptive method. This rate is higher than barrier contraceptives like condoms and is deemed to be superior to or comparable to other methods of contraception.
Terri Cornelison, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in a statement, “Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly. But women should know that no form of contraception works perfectly, so an unplanned pregnancy could still result from correct usage of this device.”
According to the company reports, over 600,000 women living in 160 countries have already subscribed to this app and three quarters of them are using it as their preferred contraceptive method. At present the app costs $80 for an annual subscription or $10 a month.