Quanterix Corporation, a company digitizing biomarker analysis to advance the science of precision health, today announced financial results for the three months and twelve months ended Dec. 31, 2018.
This past quarter has been highly productive, capping off a strong year of accelerating growth, achieving all our key milestones, and positioning us very well for 2019. Our Simoa technology is at the forefront of the biomarker revolution, which has gained considerable traction as biomarkers are increasingly being recognized for their promise to transform treatment options by accelerating the development of more effective and safer drugs for all disease categories, and monitoring patient response to treatment therapies to ensure their efficacy and safety. Longer term, we see the promise of Simoa biomarker assays being deployed as health screens to see disease very early in the disease cascade when treatments are most effective. We have been impressed with the acceleration of third-party, peer-reviewed publications and the resulting body of evidence showing the promise for Simoa blood based biomarker assays enabling early ‘pre-symptomatic’ detection of neurological diseases and cancers.”
Kevin Hrusovsky, Chief Executive Officer, President and Chairman, Quanterix
Fourth quarter 2018 financial highlights
Key financial results for the fourth quarter are shown below:
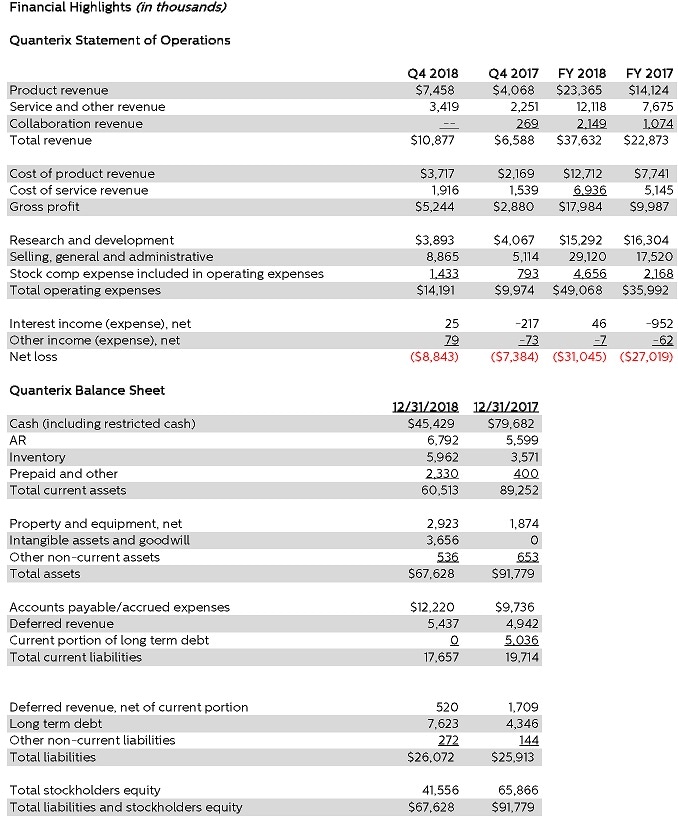
- Q4 revenue of $10.9M versus prior year Q4 of $6.6M, an increase of 65%.
- Q4 product revenue was $7.5M versus prior year Q4 of $4.1M, an increase of 83%.
- Q4 Service and Other revenue totaled $3.4M versus prior year Q4 of $2.2M, an increase of 52%.
- Q4 gross margin percent totaled 48.2%, a 450 basis point improvement over the 43.7% in Q4 2017.
YTD 2018 financial highlights
Key financial results for 2018 YTD are shown below:
- YTD revenue of $37.6M versus prior year $22.9M, an increase of 65%.
- YTD product revenue of $23.4M versus prior year $14.1M, an increase of 65%.
- YTD Service and Other revenue of $12.1M versus prior year $7.7M, an increase of 58%.
- YTD Collaboration revenue of $2.15M versus prior year $1.07M, an increase of 100%.
- YTD gross margin percent totaled 47.8%, a 410 basis point improvement over the 43.7% for full year 2017.
Excluding a one-time collaboration revenue item of $1.1M, total YTD revenue growth would have been 60%. See “Non-GAAP Financial Measures” below.
Fourth quarter and full year 2018 business highlights
- Gained unrestricted rights back for its Simoa technology in IVD markets with the termination of a license agreement with bioMérieux.
- Third-party, peer-reviewed publications continued to increase with more than 40 new publications featuring Simoa technology in Q4 alone, and >200 during the full year, bringing the total to over 400.
- Acquired Aushon BioSystems and successfully integrated the two companies. The acquisition included a CLIA certified laboratory, expanding services and accelerating entry into pharmaceutical drug trial services, as well as access to novel immunoassay technology to which Simoa sensitivity algorithms are being applied to deliver next generation Simoa capabilities.
- Adoption of proprietary Neurofilament light chain (NfL) assay, a biomarker previously measured only in cerebral spinal fluid (CSF), but by using Simoa, researchers have shown it can be measured in blood and correlated to CSF results. This advance is enabling novel applications critical for advancing early detection, treatment and prevention of neurological diseases, including multiple sclerosis (MS); Parkinson’s disease; Alzheimer’s disease; brain cancer; and traumatic brain injuries (TBIs).
- Invited by the FDA and presented on progress and the potential future for the NfL biomarker to agency officials.
- Launched a test bed, early access program for the SP-X™ Imaging and Analysis System with a 10-plex Simoa cytokine panel for oncology research and drug development.
- Increased employee headcount from 126 at Dec. 31, 2017 to 177 at Dec. 31, 2018 (>40%) and expanded the leadership team with the recent hiring of Jackson Streeter, M.D. to lead corporate development and strategy, formerly chief medical officer and chief executive officer of Banyan Biomarkers; and Mary-Ellen Cortizas to lead the Accelerator Lab, formely founder and chief operating officer of Claritas Genomics.
- Added to the Nasdaq Biotechnology Index (NBI) as part of the annual re-ranking, and attracted additional analyst coverage.
- Participated in first EU Powering Precision Health Summit, which was a highly productive meeting of leading researchers, key opinion leaders, and other stakeholders in precision health.
- Reinforced the Company’s digital biomarker position through presentations at PULSE The Atlantic Summit on Health Care, The Lake Nona Impact Forum, and MedCity Converge, as well as the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Congress, where the Company had a record number of posters/presentations mentioning the use of Quanterix’ serum NfL assay for a wide range of clinical studies in MS disease progression and monitoring.
Conference call
In conjunction with this announcement, Quanterix Corporation will host a conference call on March 7, 2019, at 4:30 p.m., EST to discuss the Company’s financial results and business outlook. To access this call, dial (833) 686-9351 for domestic callers, or (612) 979-9890 for international callers. Please reference the following conference ID: 3288482.
A live webcast will be accessible on the Investors section of Quanterix’ website: http://www.quanterix.com. The webcast will be available on the Company’s website for one year following completion of the call.