Tie layer is a new ultra-thin thermoplastic coating that can transform the medical device industry.
In this interview, Tara McCutchen, Senior Global Market Manager for Zeus, talks about their new tie layer and how it works in conjunction with FEP 2:1 heat shrink.
Tie layer is an ultra-thin thermoplastic coating applied over a PTFE liner. Please tell us about the new product tie layer and the impact it will have on the medical device industry?
Zeus now offers a new tie layer coating that can be applied onto our PTFE liners or just about any of our other tubing materials. Our standard off the shelf tie layer materials are Pebax, Tecoflex, and Vestamid and available in multiple durometers. Other materials are available upon request. The Tie Layer offers our customers:
- Improved jacket to liner bond strength (Reduced delamination)
- Class VI approved materials
- Maintain overall catheter profile
- Customized performance
- Ability to line metallic tubing
- Reduced cost/scrap
- Increased yields and throughput
- Improved patient safety
How can the tie layer product improve patient safety whilst reducing the costs?
With the addition of the tie layer, OEM’s and contract manufacturers can reduce delamination by increasing the liner to jacket bond strength up to 2.5X or higher depending on the catheter design. Improving bond strength reduces the likelihood of delamination occurring during a procedure, known as a field failure.While reducing delamination improves patient safety, it also reduces manufacturing cost by increasing yield, less inspection and higher throughput.

What makes the Tie Layer different than any other product on the market? What sets it apart?
Zeus is already known in the medical industry as a best in class polymer solution supplier with extrusion as one of our core competencies. Tie layer is a great example of our ability to take a known biocompatible material and process it in a way that allows us to further complement our critical catheter components. For manufacturers who rely on PTFE liners, tie layer offers a solution that can reduce and possibly eliminate a high cost, high liability failure potential.
 Image Credit: Photobank.kiev.ua/Shutterstock
Image Credit: Photobank.kiev.ua/Shutterstock
What risks pose a threat when it comes to catheter construction? How does Zeus help overcome these?
Delamination is one of the greatest risks in catheter construction. When different materials start to separate it limits the intended performance of that device and poses a major risk to patient safety. This defect is not easily detected and typically isn’t found until the final inspection of a fully assembled catheter. At this point, the entire catheter has to be scrapped, but even worse, a patient’s safety is at risk. With the addition of tie layer, the improved bond strength helps reduce such failures.
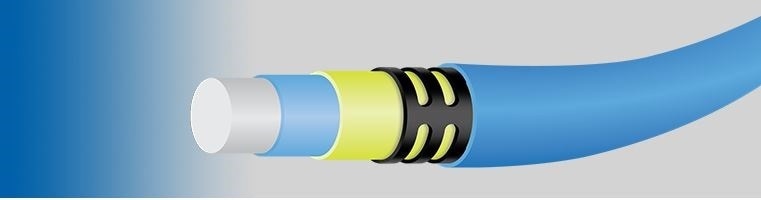
What role does FEP 2:1 play in this? What are the features and benefits of this product?
FEP 2:1 allows for reflow of catheter jackets with varying transitions. A good example of this would be reflowing over a tapered mandrel. With increased ratios, this could eliminate the use of multiple heat shrink pieces and less parts numbers for our customers. A one size fits most approach, if you will. Our 2:1 FEP HS is pure FEP and when removed it leaves a smooth surface finish.
What other applications will benefit from tie layer?
Any application that needs improved adhesion, customized performance and limited on expanding the overall profile or wall thickness. We are diligently working on several new products that will provide additional benefits from our tie layer capability…stay tuned for more new product introductions from Zeus.
What role does tie layer have in the future for the medical device market?
We believe our tie layer technology is the future for devices where adhesion and bondability are crucial to functionality. We have created tie layer solutions from the smallest of micro-catheters to the largest of structural heart catheters.
Where can our readers go to find out more?

To find out more please visit - www.zeusinc.com
Pebax is a registered trademark of Arkema France SA. Tecoflex is a registered trademark of Lubrizol Advanced Materials, Inc. Vestamid is a registered trademark of Evonik Degussa GMBH.
About Tara McCutchen
Tara McCutchen has over 15 years of experience with Zeus and promotes a collaborative approach to material selection and design for components used in the medical device industry. She shares her expertise in material science and optimized tubing solutions for a variety of applications within the medical market. Tara holds an undergraduate degree in Marketing/Management from the University of South Carolina.