A new detection method which can identify the presence of bacteria within a minute, while distinguishing healthy from non-viable bacteria, could save many lives and a lot of money. Scientists at the University of Warwick reported this technology, based on alterations in electrical signaling in bacteria in response to external electrical stimulation.
Bacterial testing is crucial to modern medical practice. Bacterial culture methods take days for results to be available. In sepsis the mortality rises by 8% with each hour of treatment delay. Similarly, up to 30% of urinary tract infections are missed on urinary dipstick studies, especially with low-level infection. Delayed diagnosis can allow infections to become established and even dangerous, causing death or disability. Delayed detection of bacterial contamination of commercial specimens also has huge economic impacts.
Against this background, bioelectrical signaling in bacteria can yield extremely useful results. The current study combined biology, mathematical modeling and engineering principles to rapidly detect viable bacteria, exploiting changes in the resting membrane potential (the baseline electrical voltage across cell membranes).
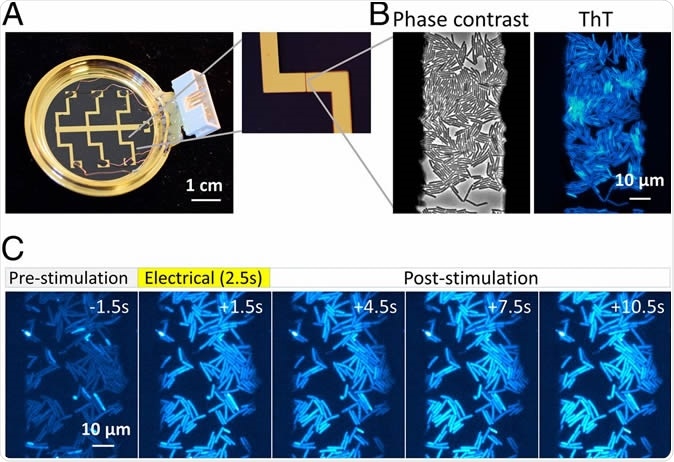
An apparatus enabling concurrent single-cell microscopy and stimulation with exogeneous electrical signal revealed hyperpolarization response to an electrical stimulus. (A) Bespoke glass-bottom dish coated with gold-titanium electrodes. Zoomed image on the Right shows 50-µm gap between electrodes. Dish is connected to relay circuit to apply electrical stimulation to bacterial cells (see SI Appendix, Figs. S1–S3 for details). (B) B. subtilis cells within the 50-µm electrode gap are visible in phase-contrast and ThT fluorescence images. (C) Film-strip images of ThT fluorescence of B. subtilis before, during, and after electrical stimulation. Increase in ThT fluorescence indicates hyperpolarization response to an electrical stimulus.
Lead author James Stratford explains, “The system we have created can produce results which are similar to the plate counts used in medical and industrial testing but about 20x faster. This could save many people's lives and also benefit the economy by detecting contamination in manufacturing processes.”
Bioelectricity in animals, which involves the study of electrical characteristics of cells exposed to external electrical fields, has been an important field of study. The study of bacterial bioelectricity is relatively new, but we now know that proliferative bacteria require a stable resting membrane potential, and use almost half their energy to maintain it. The resting membrane potential is key to electrical signaling in cells.
University of Reading scientist Yoshikatsu Hayashi comments: "Using the widely used mathematical model in Neuroscience, we revealed a common mechanism of excitable cells, neuron and bacteria cells, and the extended neuronal model could explain two distinct electric reactions of healthy and unhealthy bacteria cells. Surprisingly, a single parameter representing the degree of non-equilibrium across the membrane was sufficient to explain the distinct responses of the cells. This is an important step towards understanding the origin of electrical signalling."
Previously, both time-lapse microscopy and fluorophore-mediated single-cell membrane potential studies have been used to detect proliferative capacity. However, changes in membrane potential occur in many situations, leading to nonspecific results unless complex and meticulous calibrations are first performed. The current study looked specifically at whether the external field-induced membrane potential changes depended on bacterial proliferative potential.
The scientists used a specially-developed device to observe cell proliferation and membrane potential, and response to electrical stimulation in single cells of two bacterial species, Bacillus subtilis (B. subtilis) and Escherichia coli (E. coli).
Proliferative bacteria (observed by phase-contrast time-lapse microscopy) first took up fluorescent dye molecules as membrane voltage indicators. A 2.5 second electrical pulse was applied. The result was an intense fluorescence indicating hyperpolarization (the inside of the cell became more negatively charged than the outside).
Some of these cells were irradiated with ultraviolet (UV) light at 400 nm, which is a common bacterial growth inhibitor, and growth inhibition was confirmed by phase-contrast time-lapse microscopy. Normal cells present in different areas of the same field acted as controls. With the same stimulation, irradiated cells were depolarized (the inside became more positive) while the others were hyperpolarized. Thus this differentiated healthy from non-viable bacteria. This shift is thought to be due to a change in the resting membrane potential in damaged cells, and is predicted by the extended neuron model used in the current study.
A mixed culture of the two bacteria was then treated with vancomycin, an antibiotic which inhibits B. subtilis but not E. coli proliferation. Subsequent stimulation produced depolarization and hyperpolarization in B. subtilis and E. coli respectively. The same results were seen following treatment with ethanol or a protonophore, which also cause cell damage. This method can thus be combined with selective culture to detect antibiotic resistance.
The researchers have set up their own startup called Cytecom, and expect that commercial devices will very soon be available for industrial and clinical use, to rapidly detect live bacteria and look for antibiotic effects on bacterial cultures. Cytecom has received a grant from Innovate UK which promotes innovation.
Study author Munehiro Asally said, “It is such an exciting time to work on bio-electricity of bacterial cells. This work demonstrates that bacterial electricity can lead to societally important technology, while at the same time gaining fundamental insights into our basic understanding of cells. The tool we developed can offer more opportunities by allowing experiments which were not possible to perform before.”
The study was published in the journal Proceedings of the National Academy of Sciences of the USA (PNAS).
Sources
- Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity, James P. Stratford, Conor L. A. Edwards, Manjari J. Ghanshyam, Dmitry Malyshev, Marco A. Delise, Yoshikatsu Hayashi, Munehiro Asally, Proceedings of the National Academy of Sciences May 2019, 116 (19) 9552-9557; DOI: 10.1073/pnas.1901788116, https://doi.org/10.1073/pnas.1901788116
- Warwick.ac.uk (2019). Bacteria such as E. coli detected in minutes by new technology from Warwick University. https://warwick.ac.uk/newsandevents/pressreleases/bacteria_such_as