Anyone who has ever ordered antibodies from an antibody provider, encountered problems with antibody selectivity, specificity and reproducibility, or returned antibodies to the provider, only to receive a further low quality sample, will have suffered from the pandemic of “bad” antibodies.
It is estimated that around 50% of all antibody purchases are “bad”, accounting for approximately $350 million USD in profligate consumption in the US alone. As there are more than four million Research Use Only (RUO) antibodies available to researchers, two criteria are of critical importance: good sourcing and in-house cross-validating (where possible).
This article introduces the importance of validating the parameters (i.e. selectivity, specificity, on/off rates, equilibrium constants) of purchased antibodies, thus ensuring the receipt of a top quality antibody that is useful for the relevant applications, thus saving the user time and money.
3 Types of Antibodies
There are three principal categories of antibodies: monoclonal; polyclonal and recombinant. Polyclonal and monoclonal antibodies are produced in the immune system by memory B-cells which respond to a foreign antigen by secreting antibodies. A monoclonal antibody can recognize and bind a single epitope on a single antigen, while a polyclonal antibody possesses the capacity to recognize and bind divergent epitopes on the same antigen.
The principal difference between these categories of antibody relates to their specificity; monoclonal antibodies represent high specificity, whereas polyclonal antibodies are more likely to cause cross-reactivity, decreasing specificity as a result.
Interestingly, although the behavior of recombinant antibodies (i.e. those produced in vitro via DNA recombination, expression, purification etc.) is similar to that of monoclonal antibodies, the former exemplify a number of production advantages such as rapid production, faster turnaround and the non-requirement for a host animal. It is hypothesized that recombinant antibody technology will become the primary antibody production methodology (Bradbury, A., & Plückthun, 2015).
Issues with Antibody Quality
The last few decades have witnessed a shift from conventional small molecule drugs to antibody-based therapies and, in this regard, the demand for commercially available antibodies has increased. This has led to a rapid multiplication of new producers and distributors, paving the way for a predicted market value of more than $22 billion USD by 2025 (Grand View Research, 2017).
However, it should be noted that, as with any upward market shift this trend is likely to be followed by concerns regarding quality assurance and standardization.
The conventional production techniques of antibodies are inherently beset by batch-to-batch variability. For example, hybridomas, the vector in which monoclonal antibodies are generated, are susceptible to genetic drift. Essentially, this means that an antibody upon which a whole project has been based for years might not embody the same specificity, selectivity and/or capacity as was previously expected. This can lead to significant project inconveniences, as well as wasted time and money.
Although a number of antibody producers and distributors issue a Certificate of Analysis (CoA), Western blots and/or ELISA data, certain companies do not offer this information. Moreover, numerous producers offering antibodies which have been validated using techniques such as Western blots or ELISA will assert their suitability for a broad array of applications (i.e. IHC or flow cytometry), but will provide no supplementary documentation. Therefore, it is crucial to remain vigilant and obtain antibodies smartly and prudently.
Nevertheless, a commitment towards in-house antibody quality assurance has emerged. Validating parameters of purchased antibodies comprise sequence data and on/off rates, specificity, selectivity, and equilibrium constants. These criteria can ensure that producers are held to account, ensuring the consumer receives a top quality antibody that functions for their applications.
Binding Constants and Surface Plasmon Resonance
In a 2016 paper published in the Analytical Chemistry Insights journal,X the authors assert that, because antibody binding constants are static properties, and since only a small percentage of antibody sales are accompanied by affinity data, “it is a pity that the determination of affinity constants is rarely performed for antibodies and their respective antigens” (Weller, 2016).
In the same paper, the authors postulate that the most welcomed solution to the characterization of binding constants and the validation of antibody quality is surface plasmon resonance (SPR). Although there are alternative technologies available for characterizing binding constants, typically these approaches utilize a lot of sample, necessitate labels, generate insufficient information and/or entail substantial capital expenditure. Notably, surface plasmon resonance (SPR) has represented the paragon for acquiring quantitative binding kinetics data for a number of decades.
Significantly, OpenSPR represents a low maintenance, user-friendly benchtop SPR solution. Currently being utilized by hundreds of researchers, lab bench access to SPR technology is yielding the high-quality data that is required for successful publication. Moreover, although SPR is necessary for publications, it is also important for the development of many fields of medicine and medical research, as demonstrated by the significant increase in publications relying on SPR data, presented below.
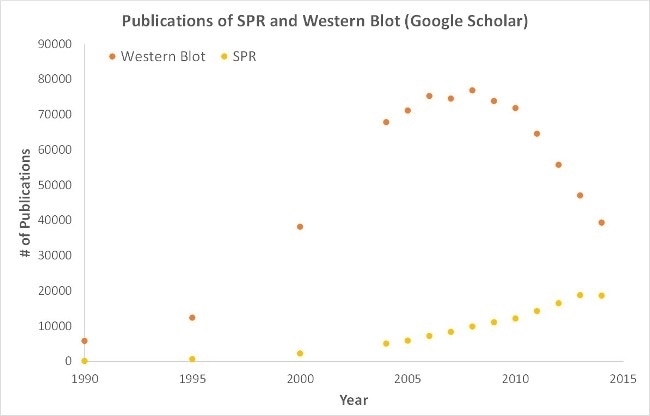 a
a
Resources
- Antibody Production Market Worth $22.6 Billion By 2025. (2017, March). Retrieved June 10, 2019, from https://www.grandviewresearch.com/press-release/global-antibody-production-market
- Bradbury, A., & Plückthun, A. (2015). Reproducibility: Standardize antibodies used in research. Nature, 518(7537), 27-29. doi:10.1038/518027a
- Weller, M. G. (2016). Quality Issues of Research Antibodies. Analytical Chemistry Insights, 11. doi:10.4137/aci.s31614
About Nicoya Lifesciences

Nicoya Lifesciences is a team of engineers and scientists with extensive experience working at the forefront of nanotechnology, biochemistry, and optical sensors.
They come from some of the world's leading research institutions such as the Imperial College of London and the University of Waterloo. They are based in Waterloo, Ontario - known as Canada's Silicon Valley.
Their mission is to bring high quality sensor products with unique, innovative designs to market to solve some of the world's most important problems. They want to drive innovation by giving more people access to powerful sensor technologies and tools.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.