After an injury, there is often damage to the nerves. These nerves usually regenerate using innate processes. Researchers from Gutenberg University Mainz (JGU) and at the Swiss University of Fribourg have now found that the Schwann cells and axons may be behind this regeneration process. The results of the study titled, “Injured axons instruct Schwann cells to build constricting actin spheres to accelerate axon disintegration,” were published in the latest issue of the journal Cell Reports.
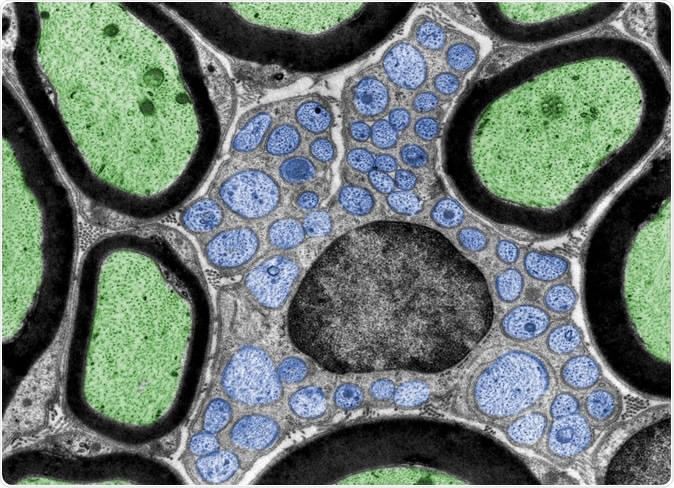
Schwann cell (in the center) containing many unmyelinated axons (blue). Image Credit: Jose Luis Calvo / Shutterstock
The study was led by Prof. Claire Jacob at Johannes Gutenberg University Mainz (JGU) who said in a statement, “An injury in the peripheral nervous system quickly triggers the activation of a fascinating repair process that allows the injured nerve to regenerate and regain its function. There is no such repair process in the central nervous system, thus injuries often lead to permanent damage such as paraplegia.” Prof. Jacob is the Head of Cellular Neurobiology at JGU. She explained that more such strategies could be developed to heal nerve injuries effectively.
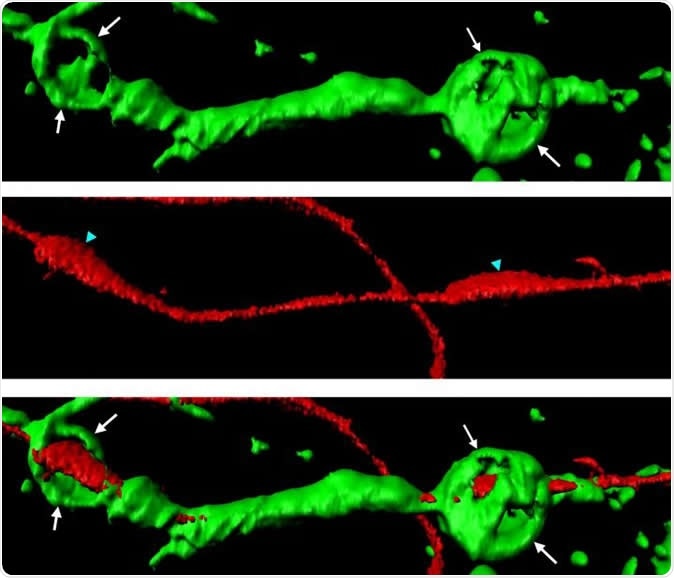
Actin spheres (green) wrapped around a severed axon (red). Image Credit: Adrien Vaquie (Cell Reports)
The team of researchers explain that each of the axons of the nerves is sheathed in myelin. Formation of the damaged myelin forms the key to regeneration of the axon. This myelin around the axon helps the nerve signals pass fast. Jacob said, “Myelin is extremely important for the function of the entire nervous system, however it also hinders the repair process in case of an injury.” The team added that in the peripheral nervous system, this myelin is produced by the Schwann cells and in the central nervous system its produced by the oligodendrocytes. Both of these types of cells have a different response to injury, the team wrote. This can may a difference in regeneration and healing of injured nerves, the team explained.
Jacob explained that the Schwann cells induce the rapid disintegration of the axons that have been damaged by the injury to the peripheral nervous system. They break the axon cells into smaller fragments that could be gobbled up either by the Schwann cells themselves or by the scavenging macrophages. The researchers explain that this removal of the debris of the damaged axon forms the first step of the repair and regeneration process. Claire Jacob said, “Schwann cells can do everything. We discovered that they not only digest myelin following injury, but they also induce the disintegration of the long axon segments that are separated from their cell bodies due to the injury.”
The team writes that the Schwann cells break down the broken and segmented axons by making small spheres of proteins called actin spheres. These spheres go on to exert pressure on the isolated segments of axon and breaks them down into smaller pieces, the team wrote. The actual need for this process is to remove the damaged part so that the healthy remaining stub of the axon could grow back and join with the other side of the broken axon to complete the neural circuitry and restore nerve function.
What was novel in this study was the find that when the axons are broken, they sent signals to the Schwann cells so that they begin making the actin spheres and start the degeneration process of the damaged axons regions. The researchers were impressed with the smoothly coordinated function of the two types of cells in nerve repair. They wrote that if there a disruption in this communication between the two types of cells, there was a slowing of axon and nerve repair process.
Next the team went on to look at the central nervous system and how the regenerating cells oligodendrocytes work there. Jacob said, “After an injury, oligodendrocytes either die or remain apparently unresponsive.” This meant that unlike Schwann cells, the oligodendrocytes do not spring into action to disintegrate the damaged axons in the central nervous system. The team investigated and found that the oligodendrocytes do not express VEGFR1 like the Schwann cells. His receptor was responsible for triggering the production of actin spheres in Schwann cells, they noted.
To take the experiment further, the team now tweaked the oligodendrocytes genetically so that these cells could express VEGFR1. They now noted that the oligodendrocytes could now produce actin structures and disintegrate the broken axon fragments just like Schwann cells. The team concludes, “These results thus identify controllable molecular cues of a neuron-glia crosstalk essential for timely clearing of damaged axons.”
At present they are working on the processes at the molecular level that could be responsible for the removal of myelin at the injury sites in the hope of reversing it. Jacob said, “We have discovered a pathway that accelerates myelin degradation in the peripheral nervous system and are now trying to determine whether this can also trigger myelin removal in the central nervous system.”
Journal reference:
Adrien Vaquié, Alizée Sauvain, Mert Duman, Noo Li Jeon, Sophie Ruff, Claire Jacob, 'Injured Axons Instruct Schwann Cells to Build Constricting Actin Spheres to Accelerate Axonal Disintegration', DOI: https://doi.org/10.1016/j.celrep.2019.05.060, https://www.cell.com/cell-reports/fulltext/S2211-1247(19)30689-8