A pair of enzymes promote non-small-cell lung cancer growth by inducing inflammation, a new study found.
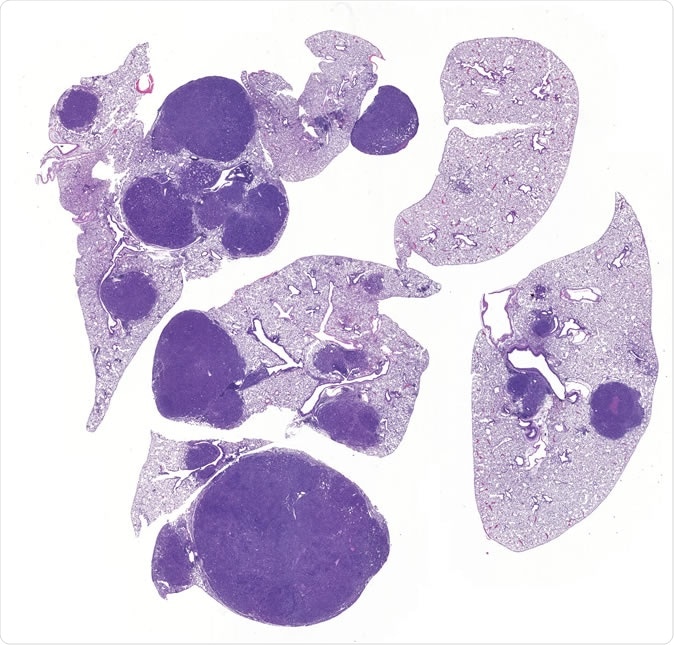
Image of lung cancer shows normal lung (light purple) and tumors (dark purple). Inactivation of SIK1 and SIK3 leads to tumor growth and inflammation, revealing for the first time that SIK kinases mediate key functions of LKB1 in preventing lung cancer. Click here for a high-resolution image. Credit: Salk Institute
Non-small-cell lung carcinoma (NSCLC) has been linked to the mutation of the LKB1 gene. The LKB1 is a serine-threonine kinase known to be involved in various cellular processes such as energy sensing, cell polarity, and signal transduction.
Some patients with this type of cancer undergo targeted genetic therapies, while others benefit from immunotherapies. However, most of the patients with NSCLC have no other treatment option except for chemotherapy. The findings of the study, which was published in Cancer Discovery, can open the doors for developing new treatment approaches for patients.
Two therapeutic targets identified for deadly lung cancer
“For the first time, we’ve found specific direct targets for LKB1 that prevent lung cancer and discovered - very unexpectedly - that inflammation plays a role in this tumor growth,” Prof. Reuben Shaw, director of the Salk Cancer Center, said in a statement.
He added that the knowledge and information provided by the study can help develop new treatment for a large number of lung cancer patients.
LKB1 acts as a tumor suppressor
A properly functioning LKB1 gene acts as a tumor suppressor, preventing the development and proliferation of cancer cells. The researchers are aware that the LKB1 gene acts as a leader, passing cellular messages to kinases, a type of enzymes. The message relay acts like a chain reaction.
LKB1 has 14 various kinases that act as teammates, but these kinases are also culprits in the development of cancer. The specific kinases responsible for carrying on LKB1’s tumor suppressive function has been unclear for the past couple of years.
In 2018, Prof. Shaw and workmates have identified the main enzymes responsible for controlling growth and metabolism. These enzymes were not as crucial to LKB1’s impact to block lung cancer, as previous experts suggested. So, they were left with 12 kinases to study, but not much is known about them, not until now.
Two kinases affect tumor growth
They found how LBK1 can communicate with two enzymes that can suppress inflammation to block tumor growth. To land to their findings, the researchers utilized CRISPR, a modest yet powerful tool for editing genomes, while allowing researchers to easily change DNA sequences and modify gene functions, combined with genetic analysis to inactivate one kinase at a time, and in combinations.
The researchers used laboratory mice in the experiment and they analyzed how inactivation in the enzymes can affect tumor growth. Hence, the researchers were left with two kinases – SIK1 and SIK3. SIK1 had the greatest effect in stopping tumor growth. But, when SIK1 was inactivated, the rate of tumor growth heightened. Meanwhile, SIK3 has also been found to affect tumor growth. In fact, when it was inactivated, the tumor grew and proliferated more aggressively.
LKB1 also plays a pivotal role in inflammation suppression in cells. The researchers found that the two kinases were particularly stopping cellular inflammation when there are lung cancer cells. The researchers concluded that when LKB1 or the two kinases are mutated in tumors, there is increased inflammation, promoting cancer cell proliferation and tumor growth.
"Discovering that of the 14 kinases it was SIK1 and SIK3 that were the most critical players is like discovering that the relatively unknown backup quarterback who almost never plays is actually one of the most important quarterbacks in the history of the sport," Shaw explained.
Future treatment for lung cancer
The study has identified a single direct route that sheds light on disease progression in lung cancer patients. The researchers have been studying about gene mutation in lung cancer since 2006 and now, they opened the doors for new therapeutic approaches for patients with non-small-cell lung carcinoma.
“This discovery highlights the nature of scientific research and how important it is to commit to pursuing difficult, complicated problems, even if it takes over 10 years to get an answer,” Shaw added.
In the future, the researchers plan to further study how the kinase-switches in inflammation can trigger tumor growth in patients with NSCLC.
Journal reference:
The AMPK-related kinases SIK1 and SIK3 mediate key tumor suppressive effects of LKB1 in NSCLC, Pablo E Hollstein, Lillian J Eichner, Sonja N Brun, Anwesh Kamireddy, Robert U Svensson, Liliana I Vera, Debbie S Ross, TJ Rymoff, Amanda Hutchins, Hector M Galvez, April E Williams, Maxim N Shokhirev, Robert A Screaton, Rebecca Berdeaux and Reuben J Shaw, Cancer Discov July 26 2019 DOI: 10.1158/2159-8290.CD-18-1261, http://cancerdiscovery.aacrjournals.org/content/early/2019/07/26/2159-8290.CD-18-1261