Neurological diseases like Alzheimer’s disease, Parkinson’s disease, depression, pain disorders and addictions could now be treated from within the brain itself using an intriguing new soft implant in the brain itself.
The new device, invented by a team of researchers based both in Korea and the United States, is capable of delivering regulatory inputs to brain circuits via a very small non-traumatic implant which in turn is controlled by, of all things, a smartphone.
The report was published in the journal Nature Biomedical Engineering. The scientists behind this invention hope that the treatment of these debilitating disorders could be accelerated by the inputs from this device. It uses replaceable cartridges capable of either administering drugs or low-energy light delivery via Bluetooth signals to treat different medical conditions. Both the light and the energy can be directed to target a desired nerve cell or cell cluster over long periods. This technique is called neuromodulation.
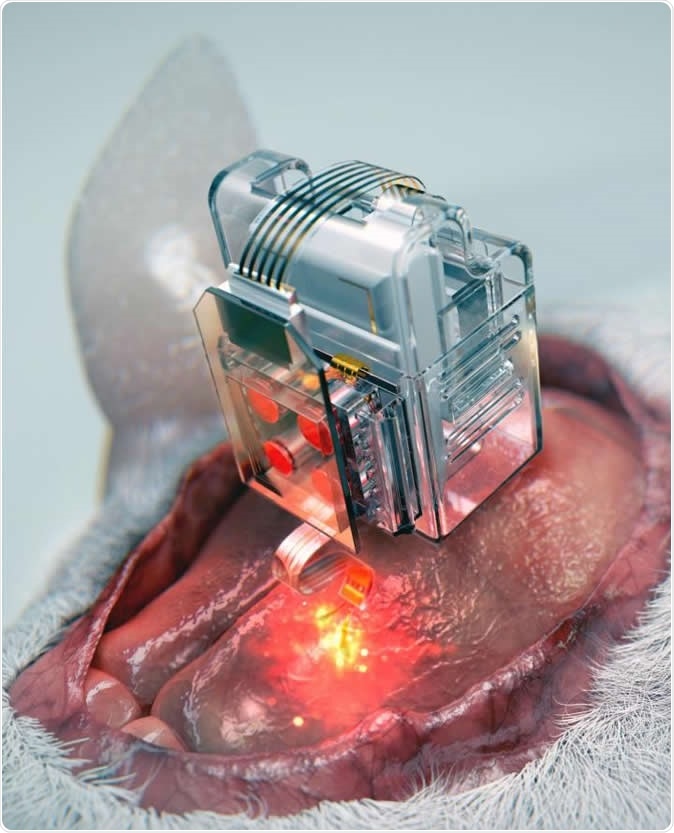
The device, using Lego-like replaceable drug cartridges and powerful bluetooth low-energy, can target specific neurons of interest using drug and light for prolonged periods. Image Credit: Korea Advanced Institute of Science and Technology
The traditional modes of modulation of nerve cells in the brain involve the use of rigid metallic tubes with optical fibers which carry drugs and light to the brain cells. As long as the device is in place, the patient’s movements are significantly limited by its physical connection to other larger pieces of regulatory equipment, posing a major issue with current devices. Another equally important problem is the brain scarring caused by the rigid nature of most devices, which causes trauma to the adjacent soft brain tissue as time passes. This means they are typically implanted for only short periods of time.
One way to reduce the incidence of brain scarring is by the use of soft probes and avoiding wired links. However, these cannot deliver drugs for more than a short period, because the drugs evaporate over time, or run out, requiring a refill. These soft probes also still required careful regulation by bulky and complicated equipment.
The present innovation could change all that. Lead author Raza Qazi explains, “The wireless neural device enables chronic chemical and optical neuromodulation that has never been achieved before.”
The basis is simple: use ready-to-go cartridges fitted to a soft and extremely thin probe, only the diameter of a human hair, to assemble a brain implant. The probe has microfluidic channels as well as very small LEDs, less in size than a single salt grain. The plug-n-play cartridges mean the implant can go on delivering drugs and light at the correct doses almost forever.
The control system is also reduced to a simple but sophisticated user interface on a smartphone. This allows scientists studying the brain to plot precise combinations of drugs and lights, which can be administered in the right order to any animal, wherever the scientist happens to be. This could also facilitate completely automated studies of animals in which the interactions between animals in terms of their reciprocal behavior can be examined. Such studies could use lights or drugs to set off a specific pattern of behavior in certain test animals, followed by observations on how this behavior affects that of the other animals.
Researcher Jae-Woong Jeong says, “This revolutionary device is the fruit of advanced electronics design and powerful micro and nanoscale engineering. “ In fact, the team behind this wants to refine the device still more so that a clinically relevant brain implant can be manufactured eventually.
Another pain scientist, Michael Bruchas, says the new technology will be of great help to researchers in multiple areas. Not only does it help to unpick the working of the brain, but it can deliver complex combinations of therapies to target specific disorders. He points out, “It allows us to better dissect the neural circuit basis of behavior, and how specific neuromodulators in the brain tune behavior in various ways. We are also eager to use the device for complex pharmacological studies, which could help us develop new therapeutics for pain, addiction, and emotional disorders.”
The scientists at Korea are involved in developing soft electronic circuits which can be incorporated into textiles and other wearable forms, as well as for implants. Meanwhile, the US scientists are studying the neural pathways that take part in regulating stress, pain, addiction, depression and other medical conditions that involve the disruption of mental health via neurological alterations. The two sets of researchers worked on this project for over three years, examining several dozen variations of the device design. They were finally able to come up with this soft implantable device which they tested in mice that were allowed to move about freely. The results of the tests showed that they could have invented a device that may actually move the study of neurological processes in health and disease a giant step forward.
Journal reference:
Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation, Raza Qazi, Adrian M. Gomez, Daniel C. Castro, Zhanan Zou, Joo Yong Sim, Yanyu Xiong, Jonas Abdo, Choong Yeon Kim, Avery Anderson, Frederik Lohner, Sang-Hyuk Byun, Byung Chul Lee, Kyung-In Jang, Jianliang Xiao, Michael R. Bruchas & Jae-Woong Jeong, Nature Biomedical Engineering (2019), https://www.nature.com/articles/s41551-019-0432-1