An important new study shows that better T cell detection is now possible using revolutionary technology. T cells are a type of lymphocyte, which in turn belongs to the diverse family of immune cells. They take part in cancer fighting as well as in most other immune responses. Recent studies have demonstrated that understanding and characterizing the T cell types that play a role in recognizing cancer cells is important in creating individualized therapies for cancer patients.
The study comes from DTU Health Technology and Jacobs University, of Bremen, and was published in the journal Science Immunology on July 19, 2019.
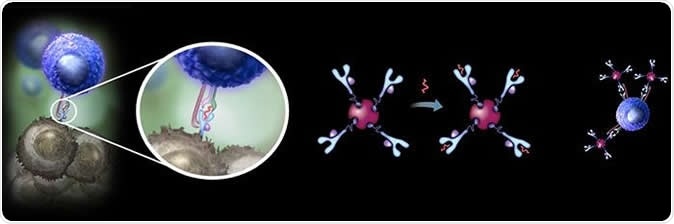
A new super-stable form of the MHC tetramer reagents, developed by Danish and German researchers, opens a range of new possibilities for improved monitoring and tracking disease relevant T cells in development of personalized cancer immunotherapies. Image Credit: DTU
T lymphocytes, or T cells as they are commonly called, have the ability to pick out both virus-infected cells and cancer cells, and then destroy them. Their capabilities are therefore crucial in the immune response to viral infections and tumors. When immunotherapy is used to treat some cancers, the aim is to stimulate the T cells in the patient’s circulation that are specific to the tumor. These tumor-specific T cells must be identified and their count monitored to find out how well the treatment is working.
This identification is carried out by a molecular dye, called an MHC tetramer. This colored compound allows scientists to visualize the T cells specific for the tumor in question, and to count them, either in microscopic fields or using a flow cytometer which allows for high-throughput assessments.
The MHC proteins have been known for a long time. These help immune cells to recognize specific antigens that denote viral infection or cancer. This is because of a site called the peptide-binding cleft which is responsible for attaching a particular tumor or viral peptide on the surface of the affected cell. The MHC-peptide complex is then easily identified by one specific clone of T cells, facilitating the elimination of that infected or cancerous cell. The attached peptide is what stabilizes the MHC molecule, and without it the molecule is intrinsically unstable. It is the presence of the specific MHC-peptide complex that is important for the activation of specific T cells as well.
These MHC-peptide complexes are common to all natural MHC proteins. However, until now, stable empty MHC tetramers were not available, since the empty (‘non peptide-loaded’) MHC molecule is too quickly degraded to synthesize in the laboratory to any meaningful extent, even when stored in the cold.
The only way out until now was to engage a commercial manufacturer for the process of production, which consumed from four to six weeks.
This delay could jeopardize urgent research deadlines or patient diagnosis. The current study dealt with this by using a new process involving disulfide stabilization (DS) of class I MHC molecules that are not attached to viral or tumor peptides. This allowed them the development of a very stable form of the MHC tetramer reagents.
The stabilization involves introducing a disulfide bond between two sulfur atoms on two separate helices, α1 and α2 helices, near the F pocket. This allows any peptide to be added at once to the MHC molecules as required by the researcher, with complete structural identity to the natural peptide-loaded molecule, except for the disulfide bond. In this way the researchers have made it possible to build up a large library of MHC-peptide reagents.
The researchers have synthesized DS-stabilized versions of three MHC molecules in this class. During tests, they were able to quickly screen T cells found within human malignant melanomas, using DS-HLA-A2 peptide multimer libraries. These libraries were built by Moritz et al., and are used to screen affinity-matured TCR to detect a cross-reaction with self-peptide-MHC complexes. The aim of the screening was to detect any specific reactions between these T cells and newly emerging antigens on the melanoma cells.
The disulfide-stabilized MHC tetramers are even better staining reagents for antigen-specific T cells compared to the wild-type or natural molecules. The researchers expect that their development of stable empty class I MHC molecules in tetramer form, that can be instantly converted to antigen-specific MHC tetramers to detect a specific T cell in just one step, will help this field to expand.
This will help detect T cells that bind to new antigens generated by tumor mutations, as well as other antigens characteristically expressed in human melanoma cells.
The lead researcher Sine Reker Hadrup says, “The technology opens a range of new possibilities for tracking disease relevant T cells in patients and to manipulate T cells to specifically fight e.g. cancer.” She feels that the stable MHC protein could help greatly to further personalized T cell treatment with patient-owned T cells that are specific for their tumor. These will be extracted from the patient’s blood, to be activated, and then they can be used to fight the tumor. This is called precision activated cell therapy.
Along with senior author Sebastian Springer, Hadrup plans to commercialize and sell this inventive product, which is in great demand by companies which are exploring new forms of immunotherapy using T cells, or new diagnostic techniques. Precision activated T cell immunotherapy could be yet another commercial application.
Journal reference:
Empty peptide-receptive MHC class I molecules for efficient detection of antigen-specific T cells, Sunil Kumar Saini, Tripti Tamhane, Raghavendra Anjanappa, Ankur Saikia, Sofie Ramskov, Marco Donia, Inge Marie Svane, Søren Nyboe Jakobsen, Maria Garcia-Alai, Martin Zacharias, Rob Meijers, Sebastian Springer, and Sine Reker Hadrup, Science Immunology 19 Jul 2019: Vol. 4, Issue 37, eaau9039, DOI: 10.1126/sciimmunol.aau9039, https://immunology.sciencemag.org/content/4/37/eaau9039