A team of researchers including those from Biotechnology Center of the TU Dresden (BIOTEC), have found that stem cells could be used for several forms of tissue engineering including tooth repair. Study leader Dr Bing Hu of the Peninsula Dental School of the University of Plymouth, published the results of the study titled, “Transit amplifying cells coordinate mouse incisor mesenchymal stem cell activation,” in the journal Nature Communications.
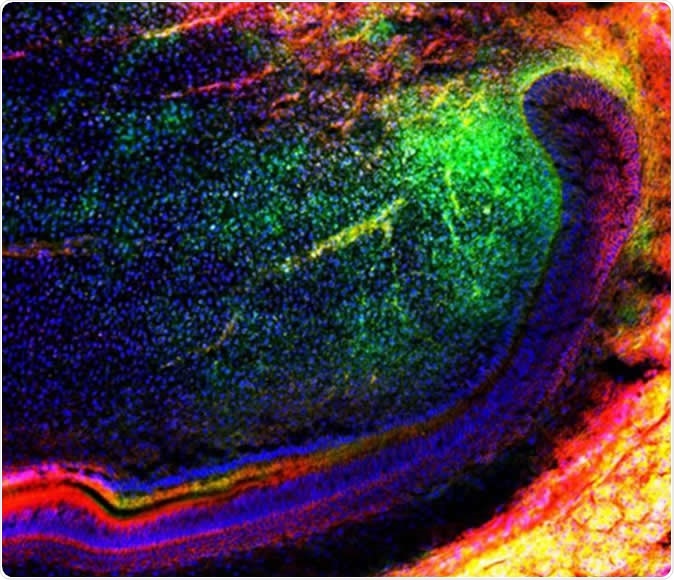
The image shows a group of mesenchymal (green) stem cells migrating in a tooth to further regenerate tissues. Source: Media and Communications | University of Plymouth
The researchers explained that stem cells have been manipulated into becoming several types of tissues and special cells in the body. In their study they coaxed these stem cells to become teeth. Tooth repair, they explain is notoriously difficult and this new technique could offer help to many patients. Hu was assisted by Dr. Denis Corbeil and his colleague Dr. Jana Karbanová from BIOTEC. Corbeil explained, “The discovery of this new population of stromal cells was very exciting and has enormous potential in regenerative medicine.”
The team explained their use of the mice incisor model because, “mouse incisors undergo lifelong growth as a result of epithelial–mesenchymal interactions. Each mouse incisor tooth has a persisting epithelial SC-TAC zone at its posterior end of the tooth, named cervical loop (CL) based on the epithelial structure.”
For their study the team noted that that mice incisor teeth contained mesenchymal stromal cells which grew consistently. In the incisor tooth model the team performed its experiments. These mesenchymal stromal cells were found to give rise to dentin. Dentin is the hard tissue that covers the tooth completely. The team noted that if these stem cells or stromal cells were activated, they sent signals to their original cells so that more number of cells are produced. This occurs with the use of a gene called Dlk1.
The team wrote that there are two types of cells that promote regeneration – “connected tissue-specific mesenchymal stem cells (MSCs) with mesenchymal transit amplifying cells (MTACs).” The team wrote that these MSCs occurred around the MTACs and the epithelial stem cells. Their key modulator, the team wrote was “Notch signalling”. This signalling from the MTACs occurred through the activation of the “Delta-like 1 homolog (Dlk1)”. They explained that Dlk1 activation was the key to maintain the balance between the MSCs-MTACs number and the differentiation of the different cells of their lineage. The Dlk1 also stimulated the epithelial stem cells and primes them for self-renewal wrote the researchers.
This new study is the first to show that the gene Dlk1 activation is the key to activation of the stem cells to produce the desired cells that would help in regeneration and wound healing in the mice models. According to the researchers, this study could lay foundation for more innovation in tooth repair and also help patients with teeth decay, tooth breakage due to injuries etc. the team hopes to translate their research into clinical applications on humans as a next step.
Related review of studies
In a recent review of studies (published in January this year), authors MS Mozaffari and colleagues from Department of Oral Biology and Diagnostic Sciences, Dental College of Georgia, Augusta University, United States, explain the uses of stem cells in tooth regeneration. Their study titled, “Stem cells and tooth regeneration: prospects for personalized dentistry,” was published in the EPMA Journal.
The team explains that use of stem cells in tooth repair and regeneration could help personalize dental treatment to a great extent. They explain that adult stem cells are being increasingly used for tissue engineering. These stem cells use scaffolding materials for support and other supporting cells to produce desired tissue systems for both experimental studies and therapy, the team writes.
The researchers add that two common problems encountered in dentistry include “dental caries and periodontal diseases.” These could be addressed using stem cells, they write. They add that pluropotent stem cells could be coaxed to prepare dental systems fro various other types of problems as well.
They conclude, “...evolving developments in the field of regenerative dentistry indicate the prospect of constructing ‘custom-made’ tooth and supporting structures thereby fostering the realization of ‘personalized dentistry.’”
Stem cell sheets
Authors L Hu and colleagues from Molecular Laboratory for Gene Therapy and Tooth Regeneration, Beijing Key Laboratory of Tooth Regeneration and Function Reconstruction, Capital Medical University School of Stomatology, Beijing, China, in June this year published their work on regenerative potential of stem cells in context of teeth.
Their study titled, “Regeneration characteristics of different dental derived stem cell sheets", was published in the Journal of Oral Rehabilitation.
The team looked at the regenerative potential of stem cell sheets that had been derived from “dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs) and stem cells of the apical papilla (SCAPs).” For this the team used PDLSCs and SCAPs from the same individual. These cell sheets were studied at the molecular level and also transplanted to special genetic strain of nude mice to see their regenerative capacity.
Results showed that there was in vitro DPSC, PDLSC and SCAP cell sheets have similar characteristics but their effects in vivo, in the nude mice, was different. The authors concluded that this showed that these stem cell sheets could be used for a variety of purposes and regeneration of different tissues as desired.
Journal reference:
Transit amplifying cells coordinate mouse incisor mesenchymal stem cell activation, Jemma Victoria Walker, Heng Zhuang, Donald Singer, Charlotte Sara Illsley, Wai Ling Kok, Kishor K. Sivaraj, Yan Gao, Chloe Bolton, Yuying Liu, Mengyuan Zhao, Portia Rebecca Clare Grayson, Shuang Wang, Jana Karbanová, Tim Lee, Stefano Ardu, Qingguo Lai, Jihui Liu, Moustapha Kassem, Shuo Chen, Kai Yang, Yuxing Bai, Christopher Tredwin, Alexander C. Zambon, Denis Corbeil, Ralf Adams, Basem M. Abdallah & Bing Hu, Nature Communicationsvolume 10, Article number: 3596 (2019), https://www.nature.com/articles/s41467-019-11611-0