Kidney failure is a lifelong burden in hundreds of thousands of people who need to be treated either by dialysis or a kidney transplant. Both procedures significantly increase the cost of healthcare and reduce the quality of life.
A common cause of this condition is autosomal dominant polycystic kidney disease (ADPKD), affecting 1 in 500 to 1,000 patients. It is among the most common genetic diseases. New research shows that simply preventing stone formation in the kidneys could potentially arrest this condition and preserve renal function. The research paper titled, 'Crystal deposition triggers tubule dilation that accelerates cystogenesis in polycystic kidney disease', is published in the The Journal of Clinical Investigation.
APKD is characterized by the progressive growth of cysts forming within the kidney, damaging the filtration tissue, and ending in kidney failure in about half of all cases. No medical treatment exists, though a new drugs has recently been approved which inhibits the activity of the vasopressin receptor. However, this agent is costly and may damage the liver in some cases, besides promoting excessive water loss from the body through urine.
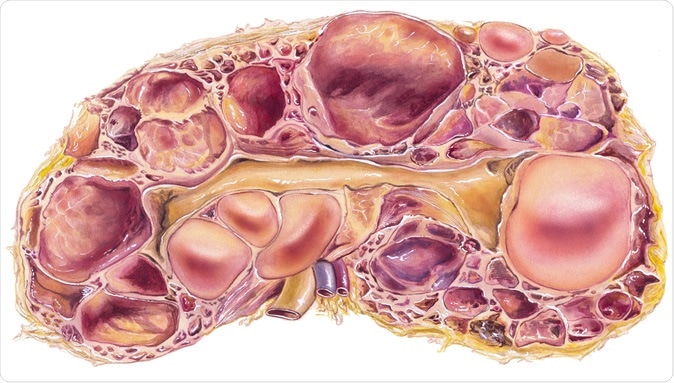
Kidney - Polycystic Disease. Autosomal-dominant polycystic kidney disease, or ADPKD, a disorder passed down through families in which many cysts form in the kidneys, causing them to become enlarged. - Illustration Credit: Medical Art Inc / Shutterstock
What causes ADPKD?
ADPKD is due to a genetic mutation in one of two genes called the PKD1 or PKD2 genes, which produce the proteins polycystin1 (PC1) and polycystin-2 (PC2) respectively. However, the exact role they play in the disease is unknown. Despite its strong genetic basis, ADPKD is inherited within affected families in very different ways, suggesting that other environmental factors also play a role.
The search for the factor that eventually drives the progression of the disease led to the theory that ADPKD is a “third-hit” disease. In this model, three insults are required to trigger the formation of a cyst in the person with ADPKD. The first two hits are mutations which damage both members of either the paired PKD1 or PKD2 genes, of which one is inherited from the parents and the other random. While the first is present at birth, the second may occur later in life, after the kidney is mature.
The third hit is another injury to the kidney, such as that caused by inadequate blood supply or a toxin, as a result of which a damage/repair sequence is switched on. In mice, for instance, the mere absence of functional PKD1 genes in mice has been proved to be inadequate to initiate cyst growth by itself. Subsequent renal injury, however, promotes rapid cyst growth.
Ischemic or toxic injury is too rare in humans to explain why ADPKD is so common, however. Thus the researchers looked at the occurrence of microcrystals in the kidney, a frequent and persistent problem in humans, to identify a possible trigger for cyst formation.
Why do crystals form in urine?
Human urine is formed within the kidneys from a supersaturated filtrate of plasma, which contains many substances capable of forming crystals such as calcium oxalate (CaOx), calcium phosphate and uric acid. In fact, millions of microcrystals do form every day. The amount of crystals is heavily influenced by diet and disease, so that up to one in four urine samples from healthy people shows the presence of obvious crystals. It is clear that the normal kidney acts efficiently to prevent crystals from growing too large and to flush them out early, keeping the renal tubules clear. This involves molecules like osteopontin, nephropontin and Tamm-Horsfall protein (THP), all of which reduce crystal formation and growth.
Sometimes crystals do manage to lodge in a renal tubule and begin to grow, or multiple crystals wash in together and join to form a larger crystal. This is called kidney stone disease or nephrolithiasis. Up to 80% of stones in humans are caused by CaOx. So far it has been thought that these crystals are removed by crossing the epithelial barrier to enter the interstitial space and either be reabsorbed by the body or form larger stones. This is too slow and tedious a process, however, to account for the rapid removal of the vast majority of microcrystals. In the current study, all microcrystals were found to be inside the tubules and not in the interstitial tissue.
Do urinary crystals cause ADPKD?
The study presents several links between crystals in urine and ADPKD. Firstly, clinical kidney stone disease is seen in 20% to 28% of patients with ADPKD. Secondly, ADPKD is more common in patients with stones. Thirdly, almost a fifth of ADPKD patients have higher than normal CaOx levels in urine, almost a quarter have gout (associated with uric acid crystals in the joints) and over 60% have high levels of uric acid, all of which are associated with oxalate and uric acid crystal formation. Uric acid crystals are linked to faster cyst growth in ADPKD. Again, like ADPKD, stones occur in males more frequently than in females.
In the current study, researchers also explored the role of crystal growth in the speed of cyst formation. They found that a previously unconsidered mechanism is probably responsible for ridding the kidney of microcrystals. This is called tubule dilation.
While tubule dilation has been observed earlier, the current study recognizes its role in protecting the kidney against crystal-induced damage by promoting the rapid passage of microcrystals in the tubules is recognized.
The renal tubules are the filtration units of the kidney, and have several parts, including the proximal tubule at the beginning, a hairpin-shaped loop of Henle with an ascending and descending limbs, and a thin part in between, and the distal tubule at the end, leading into a collecting duct to drain the filtered urine into the collecting system.
When there are CaOx crystals within the kidney, the mTOR and the Src/STAT3 signaling pathways become active, leading to the prompt dilation of the affected renal tubule along the whole length up to and including the collecting duct. It is important to note that these same pathways are active in ADPKD and promote cyst growth. The mTOR pathway regulates the size and rate of proliferation in the tubular epithelium. It also causes change in cell shape via the actin cytoskeleton, with flattening of the cells as the tubular diameter increases. It also causes the cell cycle to be arrested in G1 stage, characteristic of hypertrophy or increase in cell size rather than number. Once inactivated, the normal cell cycle is resumed.
Inhibition of the mTOR molecule causes stone formation as tubules fail to dilate, and microcrystals build up at the bottleneck where the proximal tubule ends and the thin descending loop of Henle begins. This is the thinnest part of the tubule. On the other hand, about 7 days from the start of therapy for CaOx crystals, the tubule diameter returns to normal.
In support of the hypothesis that CaOx crystals act as the “third hit’ which triggers cyst formation and growth, they found that chronic exposure to these crystals results in the deposition of CaOx crystals in male rats (but not females) with PKD, and increases disease severity. Increased dietary phosphorus acts similarly in another rat model.
Primary hyperoxaluria 1 is another genetic disease that causes the accumulation of oxalate crystals in the kidney and eventual kidney failure. Here too, mTOR /STAT3 activation is present along with dilated renal tubules. Citrate levels in urine are inversely related to disease severity in ADPKD patients.
What do we learn?
Crystals lodged within the renal tubules triggers mTOR/STAT3 signaling to cause transient dilation of the tubules as a protective mechanism. Persistent activation in ADPKD is thought to be the reason for continued tubular dilation, progressing to cyst formation. Researcher Thomas Weimbs says, “In kidneys genetically preconditioned to form these cysts, we found that these crystals can trigger the same dilation, but instead of going back to normal those tubules overshoot and form cysts."
If this is true, ADPKD patients could be treated by increasing their water intake and prescribing citrate supplements, which are known to prevent CaOx crystallization. Dietary changes are also advisable to reduce urinary oxalate, phosphate and uric acid levels. The first two steps are known to reduce cyst progression in rats with PKD, and the current study may have just told us how they work. This program, which is already in use to prevent oxalate stone recurrence, would be an easy and cost-effective way to reduce the burden of ADPKD.
Journal reference:
Crystal deposition triggers tubule dilation that accelerates cystogenesis in polycystic kidney disease. Jacob A. Torres, Mina Rezaei, Caroline Broderick, Louis Lin, Xiaofang Wang, Bernd Hoppe, Benjamin D. Cowley, Jr., Vincenzo Savica, Vicente E. Torres, Saeed Khan, Ross P. Holmes, Michal Mrug, & Thomas Weimbs. Journal of Clinical Investigation. https://doi.org/10.1172/JCI128503. https://www.jci.org/articles/view/128503/pdf